Chinese scientists find that NMN protects against intestinal infection in sleep-deprived mice and elevates an antibacterial bile salt that increases the survival of infected moth larvae.
Senior author, Dr. Yuan Liu | Revive
By Victor Ciardha Published: 10:02 am PST Feb 7, 2023 | Updated: 4:43 p.m. PST Feb 7, 2023
Highlights:
Sleep deprivation (SD) can lead to cardiovascular disease, cognitive impairment, and intestinal infection. Thus, finding ways to mitigate the adverse effects of SD is critical for health and longevity.
“According to the World Health Organization (WHO), intestinal infections annually kill more than two million people worldwide, most of whom are children,” state Fang and colleagues, the authors of a new study from Yang Zhou University in China.
Fang and colleagues have found that NMN fights against intestinal infections. The researchers report in Advanced Science that NMN ameliorates SD-induced gut bacteria changes in mice. NMN also reduces infection and increases a bile salt called DCA. They go on to show that DCA has antibacterial properties that increase the survival rate of infected mice.
NMN Protects Against Intestinal Infection
To establish an SD mouse model, Fang and colleagues used a continuously rotating machine to interfere with mice and prevent them from sleeping for three days. During these three days, 100 mg/kg of NMN was fed to the mice. To induce intestinal infection, the mice were force-fed pathogens, including a bacteria called MRSA T144. As a result, the SD mice had a 10 to 1000-fold higher fecal load of pathogens, which was prevented by NMN. NMN also reduces the inflammatory response to infection.
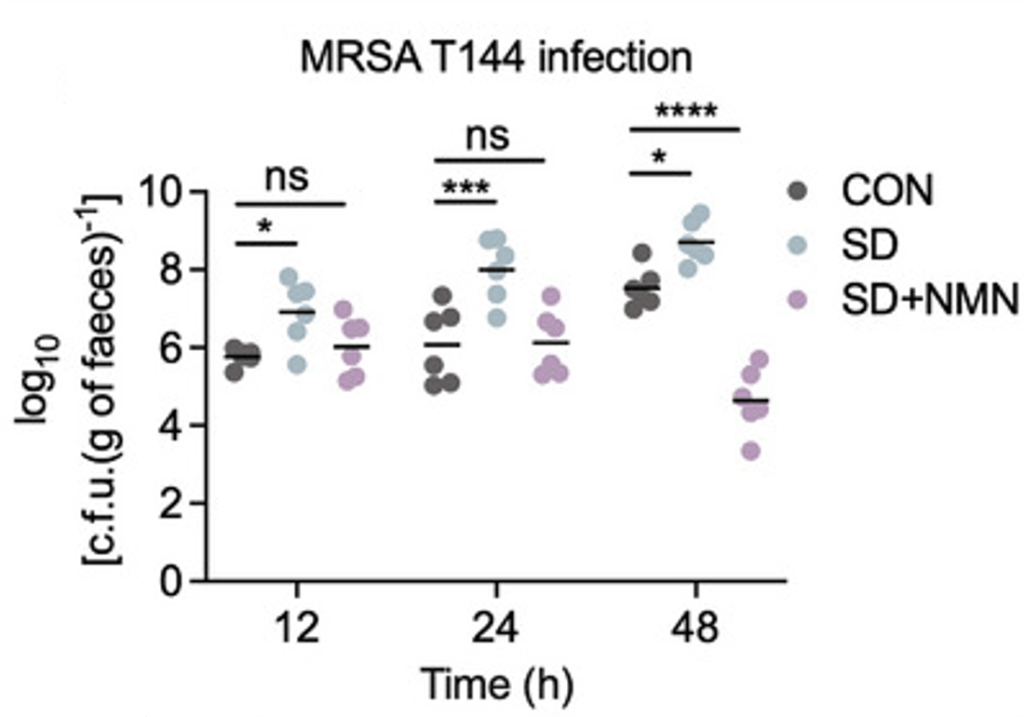
(Fang et al., 2023 | Advanced Science)
NMN Prevents Infection Caused by Sleep Deprivation (SD). Compared to normal mice (CON), SD mice have increased infection (c.f.u./g of faeces) from MRSA T144 bacteria. However, NMN (SD+NMN) prevents this infection.
To determine how NMN could reduce intestinal infection, Fang and colleagues measured gut bacteria metabolites from NMN-treated SD mice. They found that the bacterial production of a bile salt derivative called deoxycholic acid (DCA) was increased by NMN. Further experiments showed that DCA has potent antibacterial activity, and treating moth larvae with DCA for three days increased their survival by 50% when combined with the antibiotic ciprofloxacin (CIP).
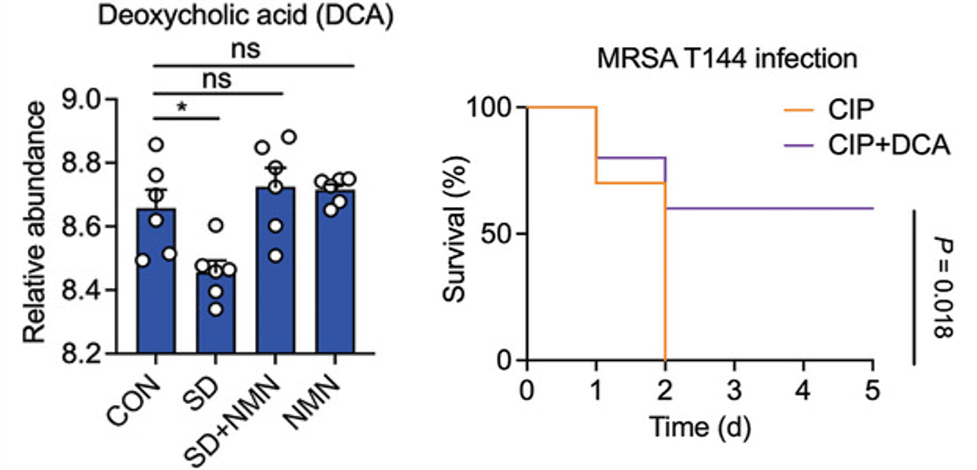
(Fang et al., 2023 | Advanced Science)
NMN Restores Life-Extending Antibacterial Metabolite Deoxycholic Acid (DCA) in SD Mice. Left:Compared to normal mice (CON), SD mice have low DCA (Relative abundance), which is restored by NMN (SD+NMN). Right: Treating MRSA T144-infected moth larvae with DCA and the antibiotic CIP increases survival by 50%.
NMN for Intestinal Health
NMN has previously been shown to restore the structural integrity of the intestine by boosting antioxidant defenses and reducing inflammation in older mice. NAD+, which is elevated by NMN, also acts as an antioxidant in sleep-deprived fly intestines, which increases their lifespan. Furthermore, NMN has been shown to improve gut bacteria composition in mice. These studies are supported by the findings of Fang and colleagues, who now show that NMN has antibacterial effects. While human studies are needed, it seems that NMN could improve intestinal and gut bacteria health from multiple angles.
Model and Dosage
Model: 8-week-old female BALB/c mice
Dose (oral): 100 mg/kg NMN during a three-day sleep deprivation period
Story Source
Fang D, Xu T, Sun J, Shi J, Li F, Yin Y, Wang Z, Liu Y. Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections. Adv Sci (Weinh). 2023 Jan 25:e2207170. doi: 10.1002/advs.202207170. Epub ahead of print. PMID: 36698264.
Scientists in China show that NMN increases intestinal mucus secretion, repairs the intestinal wall, and promotes probiotic growth in a mouse model for inflammatory bowel disease.
By Victor Ciardha Original Article: www.nmn.com
Highlights:
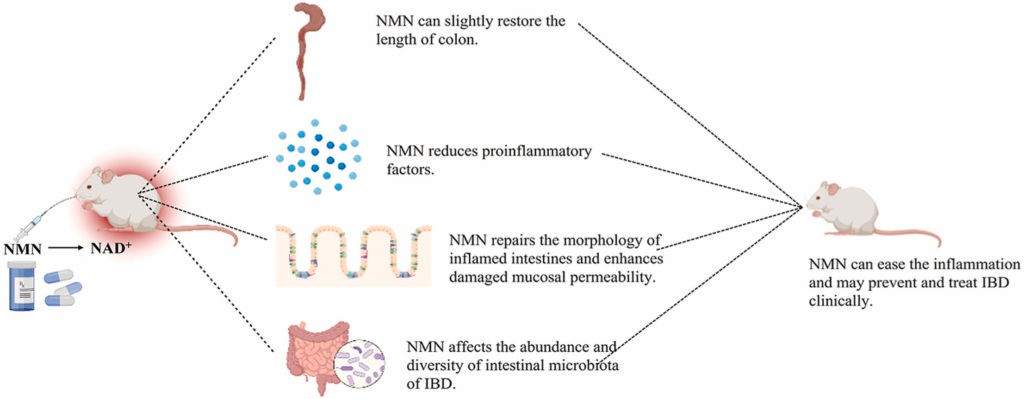
Summary of Findings. When mice modeling IBD are fed NMN, the length of their colon increases, their intestines become less inflamed, their intestinal wall becomes less permeable, and the composition of their gut bacteria improves.
Aside from pleasure, the food we eat is useless unless the nutrients it contains are absorbed through our intestinal wall. Furthermore, if our intestinal wall is leaky, unwanted molecules can sneak into our bloodstream and harm our tissues and organs. This poor nutrient absorption and leaky intestinal wall are characteristic of inflammatory bowel disease (IBD), a disease on the rise within the aging population.
Now, researchers from Jiangsu University in China report in Current Research in Food Science that NMN could treat IBD. Huang and colleagues show mice that model IBD have poor mucus secretion, heightened inflammation, and intestinal wall leakage. Feeding NMN to these IBD mice increases their mucus secretion, hampers inflammation, and repairs the intestinal wall. Furthermore, beneficial gut bacteria flourish as harmful gut bacteria dwindle when NMN is fed to IBD mice.
NMN Repairs the Intestinal Wall and Recomposes Gut Bacteria
Our intestinal wall is a barrier that prevents toxins and bacteria from entering our bloodstream. This barrier includes mucus secreted by specialized (goblet) cells. To establish a model for IBD, Huang and colleagues placed a toxic carbohydrate called dextran sodium sulphate (DSS) into the drinking water of mice. The DSS mice displayed impaired intestinal wall mucus secretion, as shown by a dye that stains mucus blue. Feeding DSS mice NMN restored much of this mucus secretion, suggesting NMN improves the protective layer of mucus lining the intestinal wall.
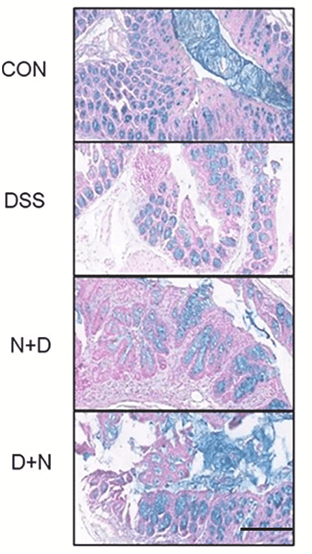
NMN Restores Intestinal Wall Mucus Secretion. Mice that model IBD (DSS) display less intestinal mucus (blue) than normal mice (CON). Treatment with NMN for 14 (D+N) and 21 (N+D) days restores much of this mucus secretion.
To test the effects of NMN on intestinal wall leakage, Huang and colleagues fed mice a small fluorescent molecule capable of permeating the gut wall. When the intestinal wall is damaged, this molecule can leak into the bloodstream and be imaged. When imaged, the DSS mice displayed body-wide fluorescence of this molecule, indicating a leaky gut. Treating DSS mice with NMN limited fluorescence to the gut region, indicating that NMN reduces gut barrier damage and permeability.
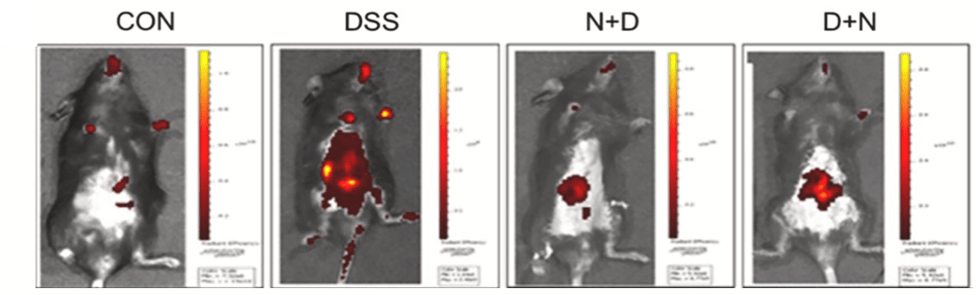
NMN Reduces Intestinal Wall Leakage. Mice modeling IBD (DSS) display high levels of gut barrier leakage (red) compared to normal mice (CON). Treatment with NMN (D + N, N +D) prevents much of this leakage, as shown by decreased red fluorescence.
Our gut contains different species of bacteria, some of which are beneficial to us and protect gut barrier function and some that are harmful to our intestinal wall. Huang and colleagues showed that DSS mice had altered gut bacteria abundance. However, treatment with NMN increased the abundance of beneficial bacteria (Firmicutes, Verrucomicrobia, Akkermansia, and Lactobacillus), demonstrating that NMN could treat IBD-induced gut bacterial changes.
NMN and the Aging Intestine
The findings of Huang and colleagues suggest that NMN could at least partially treat IBD, an inflammatory disease. Since inflammation underlies many age-related disorders, based on these results, it’s possible that NMN could also treat the decline of the intestine that occurs with natural aging. Other studies have shown that NMN reverses age-related gut barrier weakness in mice and increases the survival of mice modeling IBD. Furthermore, it was shown that NMN protects the intestinal wall by altering gut bacteria diversity. Together, these animal studies suggest that NMN can treat intestinal aging.
Source
Pan Huang, Xuxin Wang, Siyu Wang, Zhipeng Wu, Zhengrong Zhou, Genbao Shao, Caifang Ren, Meiqian Kuang, Yan Zhou, Anqi Jiang, Weihong Tang, Jianye Miao, Xin Qian, Aihua Gong, Min Xu. Treatment of inflammatory bowel disease: Potential effect of NMN on intestinal barrier and gut microbiota. Current Research in Food Science, Volume 5, 2022, Pages 1403 1411. ISSN 2665-9271. https://doi.org/10.1016/j.crfs.2022.08.011.
Japanese scientists find that NMN reduces blood vessel stiffness in middle-aged adults with higher-than-average weight and blood glucose levels.
By Victor Ciardha Original Article: www.nmn.com
Highlights:
Nicotinamide mononucleotide (NMN) is a promising anti-aging therapeutic, as it promotes the synthesis of nicotinamide adenine dinucleotide (NAD+), a molecule that declines with aging. NMN’s anti-aging potential stems from model organism studies showing that it prolongs lifespan and mitigates age-related disease. However, the degree to which NMN slows the aging process in humans remains unclear.
In a preprint article (not yet peer-reviewed) shared on Research Square, Katayoshi and colleagues from the DHC, a corporation in Japan that sells skincare products, report the findings of a clinical trial testing the effects of NMN on humans. The results showed that NMN tends to reduce arterial stiffness in healthy middle-aged adults but without statistical significance. However, statistically significant reductions in arterial stiffness were observed in individuals with above-average weight and blood glucose levels. Furthermore, NMN was calculated to reverse blood vessel aging by two years.
NMN Reduces Blood Vessel Stiffness
When young, our blood vessels are highly elastic, able to modulate blood flow to active organs. However, with age, our blood vessels lose elasticity and become stiff, making our hearts work harder. To test the effects of NMN on blood vessel aging, Katayoshi and colleagues measured blood vessel (artery) stiffness from thirty-four healthy adults aged 40-59 years who took 250 mg of NMN for twelve weeks. They assessed blood vessel stiffness by measuring how fast blood moves through an artery near the ankle. The researchers found that, while not statistically significant, NMN reduced blood vessel stiffness.
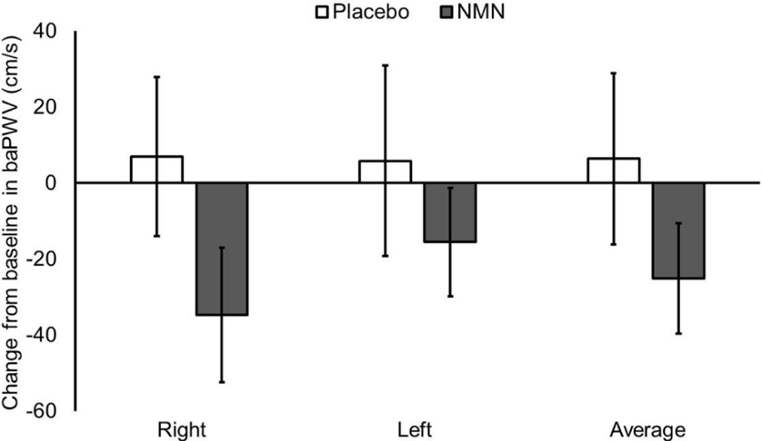
(Katayoshi et al., 2022 | Research Square)
NMN Tends to Reduce Blood Vessel Stiffness in Healthy Individuals. Participants treated with NMN (gray) have lower brachial-ankle pulse wave velocity (baPWV) values than untreated participants (Placebo) in the right and left leg, indicating reduced blood vessel stiffness.
Since high blood pressure, obesity, and high blood glucose levels are cardiovascular disease risk factors, Katayoshi and colleagues separated participants based on their blood pressure, body mass index (BMI), or blood glucose level. Participants with above-average BMI or blood glucose levels had reduced blood vessel stiffness after NMN treatment, suggesting that NMN improves vascular health in those with high BMI and blood glucose.
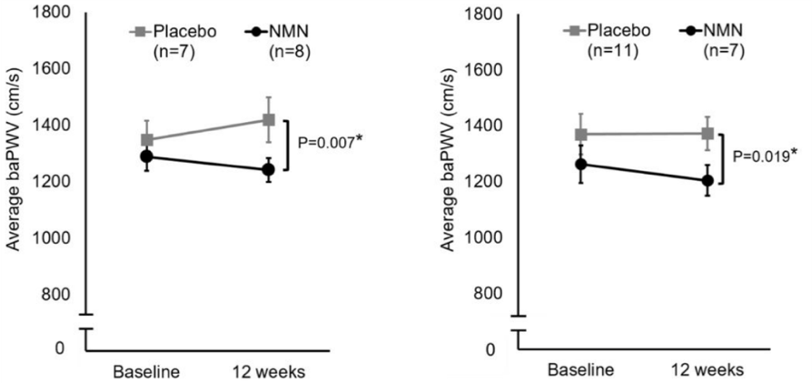
(Katayoshi et al., 2022 | Research Square)
NMN Reduces Blood Vessel Stiffness in High Weight and High Blood Glucose Individuals. Participants treated with NMN (gray) have significantly lower brachial-ankle pulse wave velocity (baPWV) values than untreated participants (Placebo) in the right and left leg, indicating reduced blood vessel stiffness.
Scientists have been able to calculate the age of our organs and tissues using various indicators, such as blood vessel stiffness. Using such calculations, Katayoshi and colleagues estimated that NMN makes blood vessels approximately two years younger in middle-aged adults. The precise process by which NMN improves blood vessel aging, however, remains unclear.
Since reduced blood vessel stiffness is associated with an increased risk of cardiovascular disease, the findings from Katayoshi and colleagues suggest that NMN supplementation could reduce cardiovascular disease risk. Only one other study has tested the effects of boosting NAD+ on blood vessel stiffness. In the study, 1000 mg of nicotinamide riboside (NR) was given to middle-aged adults for 6 weeks, resulting in a tendency to reduce blood vessel stiffness. Thus, these two studies have similar results, and it may be that a larger study could result in statistically significant findings.
NMN Reverses Multiple Aspects of Human Aging
Clinical trials testing the effects of NMN on human aging have only begun in the last few years. So far, NMN has been shown to increase insulin sensitivity, improve sleep and physical performance, enhance exercise endurance, improve skin health, increase walking speed, and increase strength in middle-aged and older adults. These clinical trials suggest that much of the research demonstrating the anti-aging effects of NMN on animals is translatable to humans. Still, more long-term studies are needed to assess adverse effects that may occur with chronic NMM supplementation. If NMN proves to be safe in the long-term, larger studies may even show more significant anti-aging effects.
Story Source
Takeshi Katayoshi, Sachi Uehata, Noe Nakashima et al. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial, 29 July 2022, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-1802944/v1]
Journal Reference
Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005 May;25(5):932-43. doi: 10.1161/01.ATV.0000160548.78317.29. Epub 2005 Feb 24. PMID: 15731494.
Several interventions have recently emerged to reverse rather than just attenuate aging.
By Jonathan D. Grinstein, Ph.D.
There’s a lot of talk these days about stopping or slowing down aging. But what about rejuvenation — the reversal of aging? Can we actually turn back the clock to become biologically younger?
Anti-aging vs. Rejuvenation
First things first: what constitutes rejuvenation, and how does it differ from anti-aging?
The line between rejuvenation therapies and other longevity interventions, such as those that slow down or prevent aging, is hazy, and these strategies are frequently used interchangeably. Anti-aging indicates the maintenance or preservation of aging biomarker status. This is typically measured by biomarkers of aging, such as clocks based on DNA modification patterns or the length of telomeres — the protective DNA caps of chromosomes.
Rejuvenation goes one step further and requires a strong, prolonged, and systemic reduction in biological age. A more important feature common to the existing and potential rejuvenation interventions is the exceptional enhancement of regenerative capacity.
The reversal of aging is inherently multidimensional: it may include a reduction in damage at the molecular level, renewed cell functionality at the cellular level, and meaningful physiological improvement at the organismal level. Some age reversal therapies may also induce lifespan extension. Along these lines, the effect at one level of biological organization is usually accompanied by connected effects at other levels.
Rejuvenation Strategies
Rejuvenation strategies have much in common with existing longevity interventions. Although most longevity interventions cannot systemically reverse biological age like rejuvenation therapies, they attenuate certain age-related hallmarks, with essential effects such as the reduced presence of senescent cells and increased stem cell pool size and functionality.
Some rejuvenation strategies have been shown to reverse epigenetic age, increase stem cell function, reverse age-related loss of eyesight, and increase the lifespan of mouse models. Interventions usually influence age-related characteristics across multiple levels, and robust measures (biomarkers) of age-related damage at one level could be used to identify putative rejuvenation interventions.
There are all sorts of strategies for rejuvenation, ranging from the relatively innocuous like exercise and taking supplements to the more “daring” like gene therapy and organ transplantation.
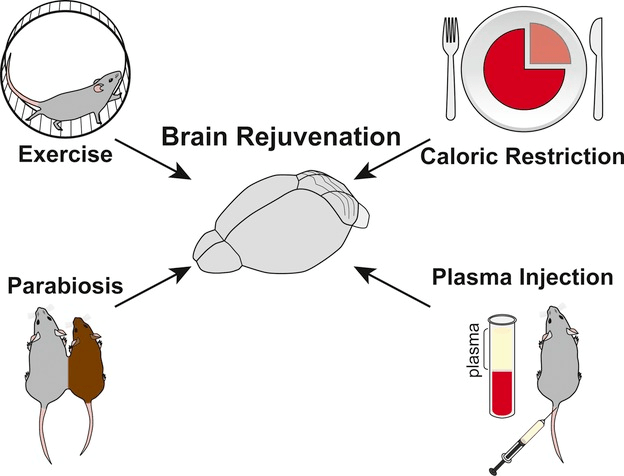
(Bouchard and Villeda 2015 | J Neurochem.)
Systemic manipulations that promote rejuvenation. Extrinsic systemic manipulations like exercise, caloric restriction, and changing blood composition by heterochronic parabiosis or young plasma administration can partially counteract aging in many organs, such as the brain.
Exercise & Other Physiological Regimens
Physical exercise increases blood delivery to most tissues and leads to changes in the systemic environment. Interestingly, numerous studies have documented rejuvenating effects of exercise on the functional and regenerative capacity of peripheral tissues and central nervous system (CNS) in animal models.
Outside the CNS, exercise can promote hematopoiesis (regeneration of blood cells) in the aging systemic environment, and increase the proliferative capacity of aged skeletal muscle stem cells. There is research showing that exercise can enhance telomerase activity to protect and even extend telomere length.
For people who cannot exercise, there are other activities that can influence systemic rejuvenation. For example, another study showed that hyperbaric oxygen therapy – exposure to 100% oxygen at elevated environmental pressure to optimize body tissue oxygen absorption – extends telomeres 20-38% in different immune cell types and reduced the senescent cell population 11-37%, depending on the type of cell.
Caloric restriction
Another systemic manipulation shown to counteract the age-induced effects on tissue regeneration is caloric restriction, a reduction of 20–40% of caloric intake without malnutrition. Caloric restriction has been shown to rejuvenate tissue regeneration in aged organisms, similar to the effects of exercise. A number of studies have shown rejuvenating effects of caloric restriction on the decline of hematopoietic stem cell function.
Rejuvenation of regeneration was also observed in skeletal muscle and on intestinal stem cells. The effects of both short-term and long-term caloric restriction on rejuvenation of regeneration are also found in the central nervous system – the brain and spinal cord.
NAD+ Homeostasis
Supplementation through NAD+ precursors in specific settings may also reverse hallmarks of aging, such as improving telomere length. For example, Increasing NAD+ levels has been shown to not only protect but also improve telomere length. One study in mice showed that the NAD+ precursor nicotinamide mononucleotide (NMN) treatment improved the length of telomeres in mice.
Senolytics
There is increasing evidence of the detrimental role of senescent cells in aging. Some of the hallmarks of aging (mitochondrial dysfunction, deregulated nutrient-sensing, loss of proteostasis, epigenetic alterations, telomere attrition, and genomic instability) induce normal cells to become senescent, which in turn can induce paracrine senescence in nearby normal cells through senescence-associated secretory phenotype (SASP). Senescence-promotion through SASP together with a decline in the immune system activity, converge to induce organismal accumulation of senescent cells.
In aged individuals, chronic accumulation of senescent cells contributes to tissue dysfunction and increased risk of age-associated diseases development. Clearance of senescent cells has improved age-associated pathologies in animal models, leading to promising new clinical trials. Different mechanisms of senescent cells can be exploited pharmacologically to develop new therapeutic targets. Senescent cells elimination with different senotherapeutic approaches can improve healthspan in aged individuals.
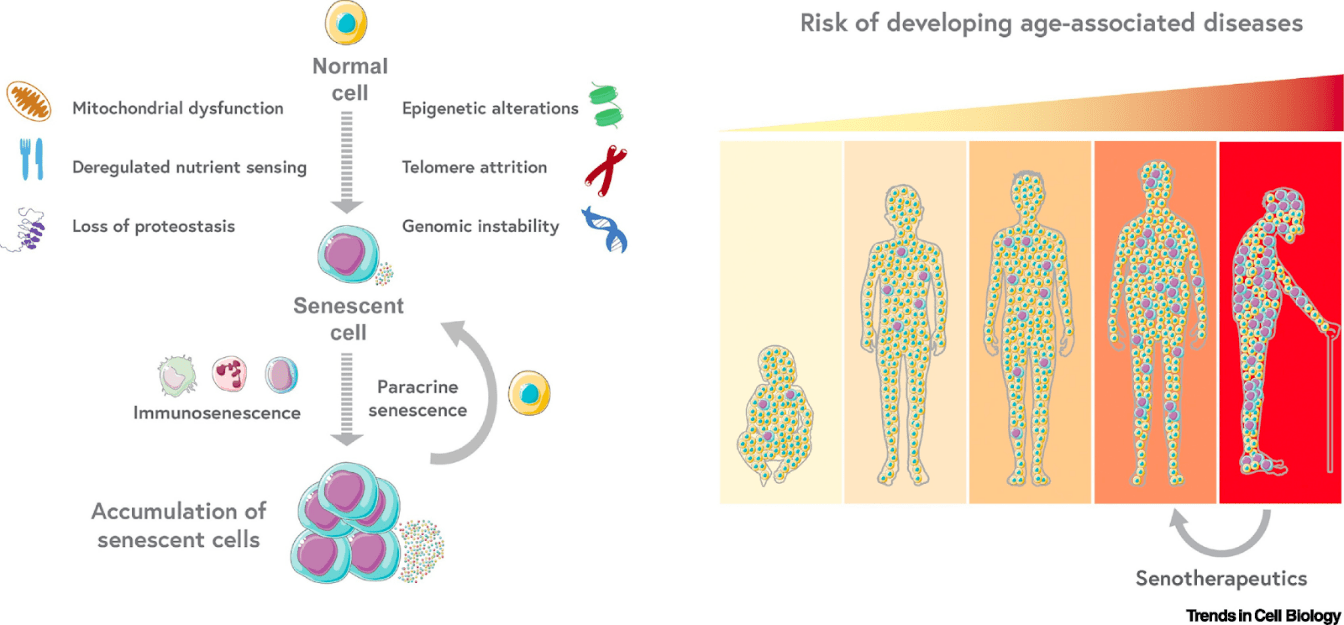
(Borghesan 2020 | Trends Cell Biol.)
Senescence-Centric View of Aging. Cellular senescence is a state of stable cell cycle arrest associated with macromolecular alterations and secretion of proinflammatory cytokines and molecules. Senescent cells are potential contributors to the age-associated loss of regenerative potential and are implicated as a central regulatory mechanism of the aging process. Exploiting mechanisms by which senescent cells drive aging and diseases can serve as therapeutic targets.
Telomere Extension
Aging is linked to shortening telomeres. This happens, in part, because of insufficient activity of an enzyme called telomerase reverse transcriptase (TERT) that maintains telomere length. Animals deficient in TERT have shorter telomeres, shorter lifespans, and an increased risk of age-related diseases like heart disease. Recent studies on animal models have shown the therapeutic efficacy of TERT in increasing healthy longevity and reversing the aging process.
Telomeres can be extended in various genetic, pharmacological, and physiological means, typically by activating TERT. For example, one screen identified small molecules that activate TERT and extend telomeres.
Another major route to elongate telomeres has been through gene therapy with TERT, either by injection or even intranasal administration. For example, a study that used safe and effective viral TERT gene therapy strategies increased telomere length in the heart, liver, kidney, brain, lung, and muscle in 2-year-old-mice by six times than in control mice of the same age. Notably, the mice treated with TERT gene therapy demonstrated healthier aging and extended longevity.
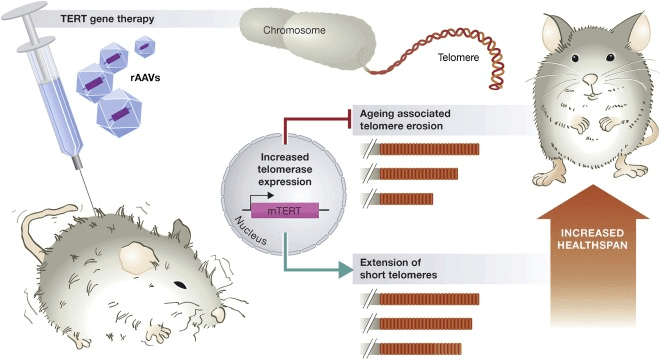
(Boccardi and Herbig 2012 | EMBO Mol Med.)
Rejuvenation in mice using a telomerase gene therapy. Delivery of telomerase (TERT) using adeno-associated viruses (rAAV) suppresses aging-associated telomere erosion and extends short telomeres in various mouse tissues. Consequently, animals display rejuvenation, improving healthspan and extending lifespan.
Cellular reprogramming
On a microscopic scale, the most extreme case of heterochronic transplantation is somatic cell nuclear transfer, which has emerged as the basis for modern-day cloning approaches (Gurdon et al., 1958). By transferring an adult cell nucleus to a de-nucleated oocyte, a new individual can be generated. This technique encapsulates the full potential of reversing the biological age of a somatic cell to that of the new embryo. Interestingly, this implies that methylation patterns in the transferred nucleus are likely to reset by cytosolic components in the oocyte, suggesting another potential mechanism for rejuvenation.
To recapitulate this effect without the introduction of complex microscopic procedures, scientists discovered four critical “reprogramming factors” (Yamanaka factors), which, when expressed in somatic cells, could effectively reverse the developmental status to that of early embryos, generating induced pluripotent stem cells (iPSCs).
Interestingly, low epigenetic ages around zero were predicted when epigenetic clocks were applied to iPSC samples. Almost all clocks showed considerable epigenetic age decreases compared with dermal fibroblasts used as the source of fully reprogrammed iPSCs.
Similar characteristics were observed in mice. In the case of mice, clocks reported a range of epigenetic ages of the identical iPSCs. Taken together, most human and mouse clocks reach a consensus in establishing age reversal that occurs as a result of reprogramming, although consistent predictions of age in these cells across different models remain a challenge.
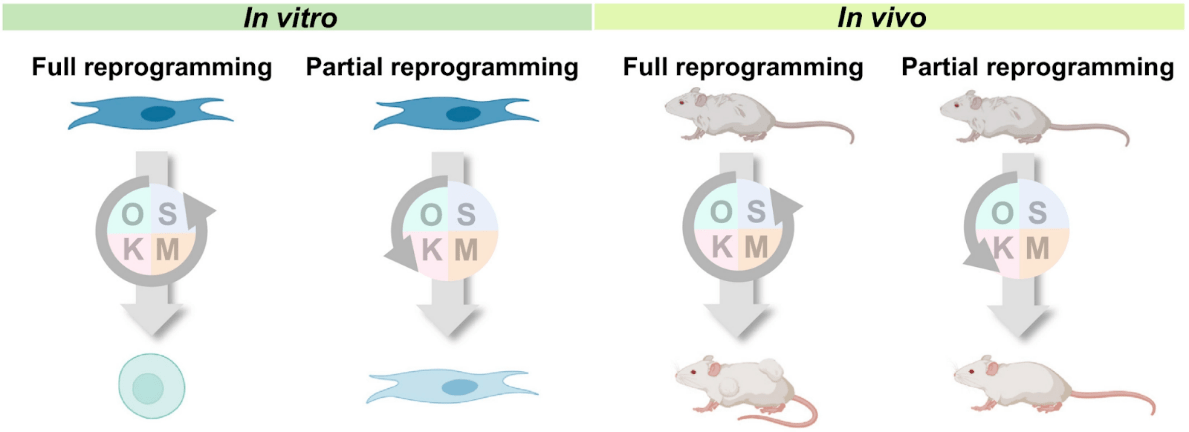
(Zhang et al., 2021 | Aging Cell)
Reprogramming approaches for rejuvenation. Schematic of reprogramming approaches for rejuvenation in cell culture (in vitro) and animals (in vivo). Full reprogramming of cells in vitro can reverse biological age to that of the embryo, but this approach can be tumorigenic in vivo. Partial reprogramming could reverse the cell’s biological age without an irreversible change of cell identity, and the in vivo approach may be promising to achieve rejuvenation.
Heterochronic transplantation
Since the 1960s, and perhaps even earlier, researchers have been transplanting tissues and organs from animals of one age to animals of different ages. These “heterochronic” age chimeras have shown rejuvenating properties.
Of the potential rejuvenation therapies that remain to be thoroughly characterized, linking the circulatory systems between a young and old organism – heterochronic parabiosis – is one of the most notable. This surgical procedure has been performed for years on rodents, and it was shown that mouse lifespan could be extended by linking the circulatory system of an old mouse with that of a young mouse.
Heterochronic parabiosis was rediscovered as one of the most promising rejuvenation interventions in 2005. By briefly connecting the circulatory system of young and aged mice, old mice exhibited youthful features in the brain, muscle, and liver, characterized by increased cognitive function, replenished stem cell pools, and augmented regenerative capacity.
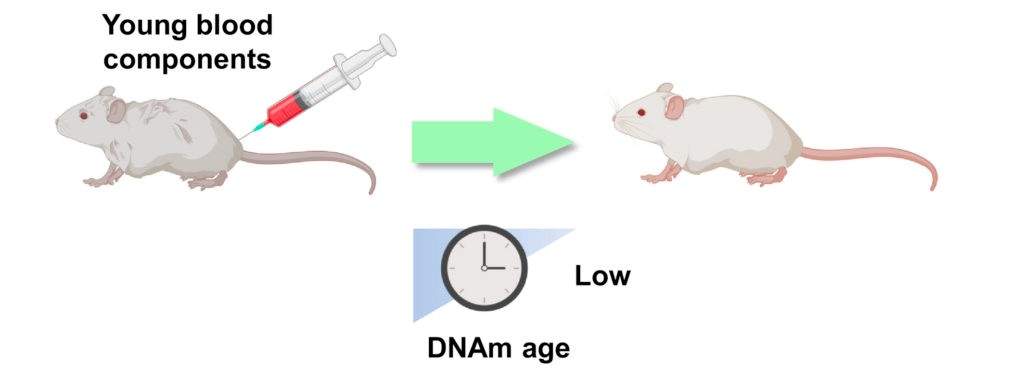
(Zhang et al., 2021 | Aging Cell)
Damage dilution in rejuvenation. In heterochronic transplantation, the damage accumulated with age is likely diluted by donor tissues (i.e., young blood), resulting in lower DNA age, as calculated by DNA methylation (DNAm) readouts. Bulk and single-cell clocks may be used to assess biological age readouts resulting from these phenomena.
Following up on this study, researchers have focused on blood components for biological age reversal, while others have investigated the transplantation of other organs to replace aged tissues. With bone marrow transplantation, the blood epigenetic age of the recipients generally matches the age of the donors, although whether this effect is systemic has yet to be established. Of note, bone marrow transplantation has also shown a 12% increase in mouse lifespan.
Additionally, a study using an undisclosed plasma fraction demonstrated robust reversal of epigenetic age, further suggesting that heterochronic transplantation may be a potential rejuvenation intervention. Recent work has also revealed that young splenocyte transplantation ameliorates the aging features of progeroid animals. In addition, transplantation of embryonic brain tissues has shown potential for neuronal repair, and ovarian transplantation was reported to improve health parameters and result in an extended lifespan.
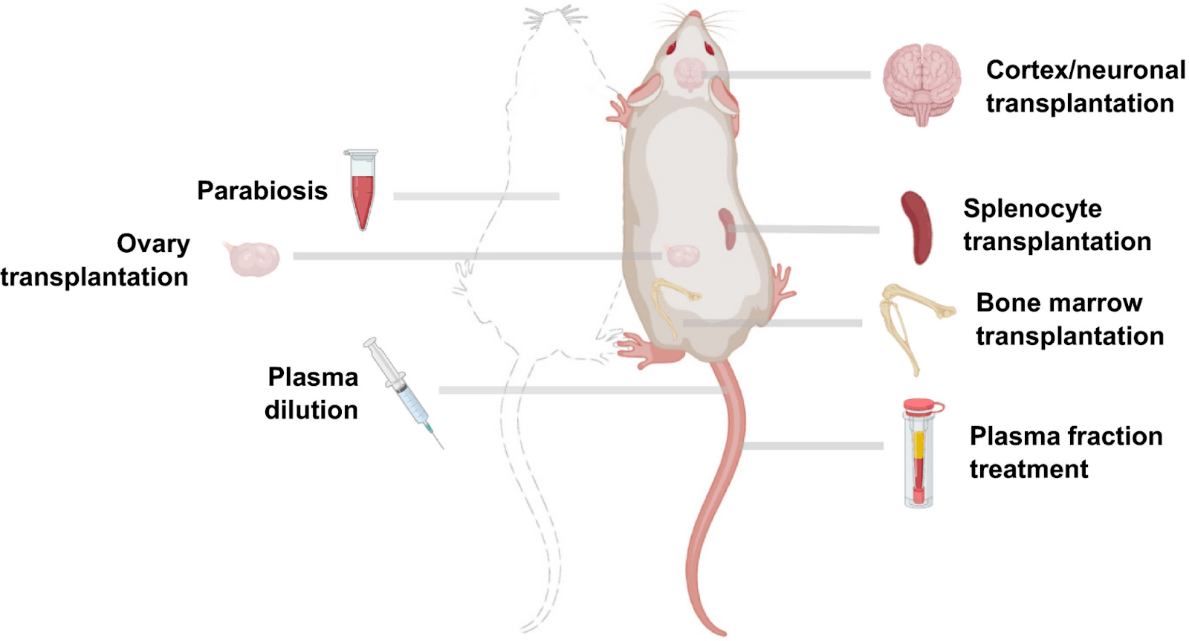
(Zhang et al., 2021 | Aging Cell)
Potential heterochronic transplantation interventions for rejuvenation in mice. Schematic of potential heterochronic transplantation interventions for rejuvenation in mice.
The Future of Rejuvenation
Overall, rejuvenation procedures provide promising prospects to reverse people’s biological ages, thereby prolonging life and improving health. However, many of the biological underpinnings of rejuvenation remain unknown, and the present adverse effects generated by some of these therapies (especially reprogramming) prohibit their widespread implementation. Existing methods, such as lowering the dose and regularly monitoring key statistics, can assist to mitigate these side effects.
However, in the future, extensive multi-modal assessments of the common aspects of present and emerging rejuvenation therapies will be important to remove these detrimental consequences. It will eventually be feasible to assess and harness the underlying links between age-reversing techniques as more of these therapies are created and independently validated based on molecular and physiological aging biomarkers.
Finally, our work at the intersection of aging, molecular profiling, high-resolution methods, and physiological assessments may pave the way for the safe and successful use of systemic rejuvenation medicines in people.
References
Boccardi V, Herbig U. Telomerase gene therapy: a novel approach to combat aging. EMBO Mol Med. 2012 Aug;4(8):685-7. doi: 10.1002/emmm.201200246. Epub 2012 May 15. PMID: 22585424; PMCID: PMC3494068.
Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol. 2020 Oct;30(10):777-791. doi: 10.1016/j.tcb.2020.07.002. Epub 2020 Aug 13. PMID: 32800659.
Bouchard J, Villeda SA. Aging and brain rejuvenation as systemic events. J Neurochem. 2015 Jan;132(1):5-19. doi: 10.1111/jnc.12969. Epub 2014 Dec 5. PMID: 25327899; PMCID: PMC4301186.
Zhang B, Trapp A, Kerepesi C, Gladyshev VN. Emerging rejuvenation strategies-Reducing the biological age. Aging Cell. 2021 Dec 31:e13538. doi: 10.1111/acel.13538. Epub ahead of print. PMID: 34972247.
Research shows that taking nicotinamide mononucleotide (NMN) improves intestine bacteria composition and telomere length.
By Brett J. Weiss.
Highlights
As people get older, the repeated DNA sequences at the ends of our chromosomes called telomeres shorten. The erosion of telomeres leads to disorders associated with aging, like metabolic disease and heart complications.
Along these lines, inflammation from aging disrupts the balance between helpful and harmful gut bacteria, leading to inflammation that precedes age-related diseases (inflammaging). Previous studies have indicated that the nicotinamide adenine dinucleotide (NAD+) precursor, NMN, lengthens telomeres and restores healthy gut microbes. Whether NMN provides these benefits for people has not yet been examined thoroughly with clinical trials.
Wu and colleagues from the Tianjin Institute of Industrial Biotechnology have shown in a recent publication from a clinical trial that administering 500 mg/L of NMN in drinking water to 16-month-old pre-aging mice (equivalent to 45-60 year-old people) for 40 days shifted gut microbiome diversity and increased telomere length.
The China-based team also found that oral NMN supplementation doubles telomere length over 90 days in human blood cells called peripheral blood mononuclear cells (PBMCs). Given these findings, it is possible that increasing telomere length may inhibit the onset of age-related diseases to thus enhance healthspan, which may also potentially increase lifespan.
NMN Boosts Metabolism in Mice
To establish the benefits of NMN given to mice, Wu and colleagues measured thermogenesis, the output of body heat, which correlates to increased cell energy production. Thermogenesis is a biomarker, a biological indicator, of metabolism — the generation of energy through cellular processes that produce the energy packet molecule adenosine triphosphate (ATP). The assessment of metabolism through body heat showed that administering NMN over a 40-week time course increases thermogenesis by about 10%, confirming that the compound improves metabolic function.
NMN Alters Intestinal Bacteria Diversity
Wu and colleagues next measured fecal bacteria composition in mice and found that NMN promotes a healthy gut by improving bacterial composition. Intestinal bacteria health is a biomarker of overall physiological well-being. The Tianjin researchers observed that short-term NMN administration reduced the diversity of bacterial species in the intestine. Notably, several species of healthy gut-associated bacteria species increased in abundance, like Mucispirillum, Colidextribacter, and Candidatus_Saccharimonas. On the other hand, bacteria often found in patients with age-related diseases like Staphylococcus, Corynebacterium, and Paenalcaligenes diminished in prevalence.
Oral Usage of NMN Drives Telomere Extension
Wu and colleagues then measured the length of telomeres in mice and humans. The team found that administering NMN for short time periods increases telomere lengths 20-25% in mice. The researchers also found that taking NMN for short periods of time (90 days) almost doubles the length of telomeres in humans, indicating potentially breakthrough health benefits and lifespan extension effects.
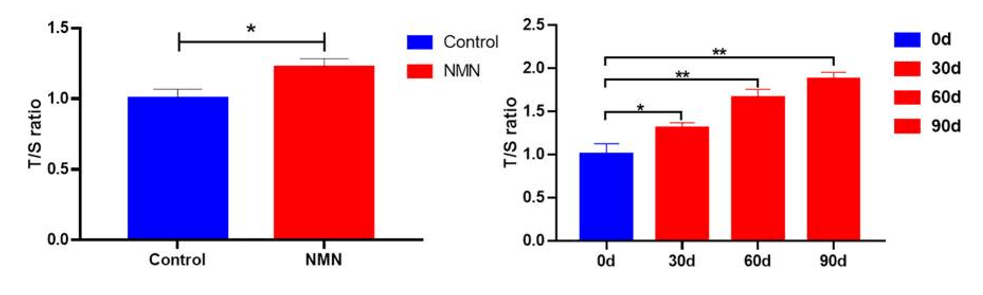
(Wu et al., 2021 | Frontiers in Nutrition)
Administering NMN to mice and people substantially extends telomere length in immune cells. The left figure shows telomere length increased by about 20% in peripheral blood mononuclear cells (PBMCs). The figure on the right illustrates that telomere lengths linearly increase such that by 90 days of orally taking NMN, telomere length almost doubled.
The evidence gathered from this research study provides insight that NMN improves gut microbial composition in mice and telomere length in mice along with people, and these effects may drastically improve healthspan. Whether or not NMN promotes a longer lifespan, the study indicates that it might help people extend the number of years they live in health and wellbeing.
Limitations to the study include that long-term NMN supplementation was not examined. The way that the body metabolizes NMN, especially in higher dosages, must be checked, also. Furthermore, what effects a lower diversity of gut flora may have during aging will require further investigation. In essence, a concern that researchers should confront soon is “how much NMN is too much?” Until the research community has a better understanding of the recommended dosages for NMN and appropriate clinical guidance, the proper amount of NMN to be consumed in humans for certain clinical indications has yet to and is currently being explored.
References
Niu KM, Bao T, Gao L, Ru M, Li Y, Jiang L, Ye C, Wang S, Wu X. The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Front Nutr. 2021 Nov 29;8:756243. doi: 10.3389/fnut.2021.756243. PMID: 34912838; PMCID: PMC8667784.
Just like the genomes in the nuclei of our cells, these energy-generating structures have their own set of DNA, which is a proxy measure for mitochondrial function and has been associated with several aging-related diseases.
By Brett J. Weiss
Highlights
Our cells contain more than one set of DNA: one in our nucleus encodes most cellular processes and another in the energy-generating structure of the cell known as the mitochondria. Just like the DNA in the nuclei of every cell, the replication and integrity of mitochondrial DNA (mtDNA) is critical for cells to function and flourish and for us to fulfill a healthy and long life. But we have a lot to learn about the link between mtDNA and mitochondrial function in the context of aging and longevity. Doing so can enable us to bolster the activity of this crucial cell structure and integrity of its separate genetic hard drive for improving healthspan and lifespan.
Kang and colleagues from Kyushu University in Japan reported in the Journal of Biochemistry that nicotinamide mononucleotide (NMN) enhances mtDNA replication. The researchers, who wrote on behalf of the Japanese Biochemical Society, found that treating human kidney cells with NMN activated and increased the rate of mtDNA replication by increasing the number of building blocks of DNA (nucleotides) in mitochondria while decreasing their degradation products (nucleosides). These findings suggest a mechanism for how NMN benefits mitochondria, metabolism, and, ultimately, aging.
The Role of NAD+ in mtDNA Replication
In cooperation with many nuclear-encoded proteins, mtDNA genes contain the instructions to components for essential cell processes, such as generating energy. Mitochondrial DNA copy number, a measure of the number of mitochondrial genomes per cell, is a minimally invasive proxy for mitochondrial function and has been associated with several aging-related diseases and all-cause mortality. However, we still only know a little about how metabolism inside mitochondria affects mtDNA maintenance and replication, let alone how these processes may underlie aging and longevity.
To address this problem, Kang and colleagues created a new method for measuring metabolism within mitochondria to understand how mitochondrial metabolism regulates mtDNA replication. With this method, which they called SLO. The Japanese research team genetically manipulated levels of an enzyme called ‘twinkle’ — a component of mtDNA replication machinery that can increase the number of mitochondria.
By increasing mtDNA copy number through twinkle manipulation, Kang and colleagues found that several components related to metabolism changed, including increases in nucleotides and NAD+ — a vital molecule that serves as an essential factor for innumerable cell processes, including mitochondrial energy generation. Kang and colleagues speculate that these results suggest that nucleotide and NAD+ increases may result from increased demand for components required for mtDNA replication.
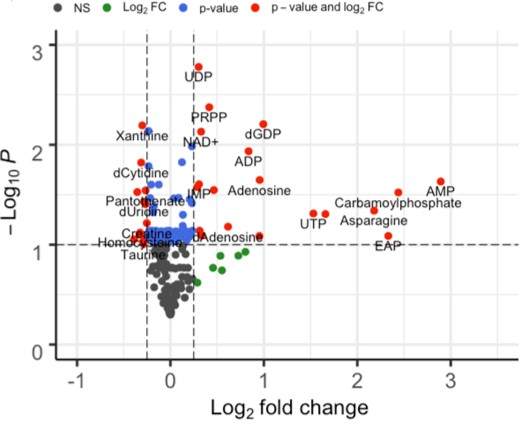
(Kang et al., 2021 | Journal of Biochemistry)
Mitochondrial DNA replication alters the levels of nucleotides and NAD+. This plot shows how the level of metabolites changed during controlled activation of mitochondrial DNA (mtDNA) replication through a gene called Twinkle. Some notable nucleotide metabolites in these results are related to adenosine (AMP, ADP) and uridine (UTP, UDP) as well as phosphoribosyl diphosphate (PRPP), which is essential for the generation of nucleotides. Red, large significant changes; blue, small significant changes; green, insignificant changes; black, no change.
NMN Modulates Mitochondrial Replication
Kang and colleagues then examined the nature of the relationship between mtDNA replication and NAD+. To show that NAD+ causally influenced mtDNA and was not just correlative, the researchers treated human kidney cells with NMN, a precursor of NAD+, for three consecutive days and then quantified the amount of mtDNA.
In NMN treated cells, the amount of mtDNA increased to levels comparable to a condition activating mtDNA replication by controlling the ‘twinkle’ protein that executes this mtDNA copying process. After establishing that NMN administration can increase the amount of mtDNA, Kang and colleagues showed that NMN also increases the rate of mtDNA replication. Combining NMN administration and twinkly activation enhanced the beneficial effects on mtDNA replication and copy number.
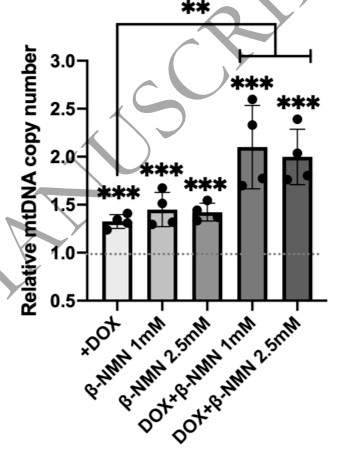
NMN enhances mtDNA replication. Kang and colleagues compared the amount of mitochondrial DNA (mtDNA copy number) in conditions where the mtDNA replication protein twinkle was activated (DOX), NMN was administered, and the combination of these two. These results show that relative to untreated cells, all conditions show increases in mtDNA number. The actions of twinkle activation together with NMN have a combined effect.
Kang and colleagues conclude the article by discussing how these data show that NMN and mtDNA replication are functionally linked through the regulation of nucleotide pool synthesis. They indicate that they are further investigating this issue to clarify the underlying mechanism.
References
Nomiyama T, Setoyama D, Yasukawa T, Kang D. Mitochondria Metabolomics Reveals a Role of β-Nicotinamide Mononucleotide Metabolism in Mitochondrial DNA Replication. J Biochem. 2021 Dec 4:mvab136. doi: 10.1093/jb/mvab136. Epub ahead of print. PMID: 34865026.