Endurance exercise is associated with reduced senescent cells – non-proliferating cells that evade programmed death and emit inflammatory molecules – in the colon of aged runners.
By Brett J. Weiss https://www.nmn.com/news/new-washington-university-study-intensive-endurance-exercise-rids-gut-of-harmful-cells
Highlights
Since ancient times, regularly exercising has been one of the key pillars to maintaining overall good health and aging gracefully. However, to this day, the underlying cellular processes by which exercise confers health benefits remain unclear. Because senescent cell accumulation can contribute to cancer and aging, researchers have turned to a cancer-prone organ – the colon – to help uncover how exercise promotes healthy aging in older adults.
Published in NPJ Aging, Fontana and colleagues from the Washington University School of Medicine show that intensive endurance exercise confers reduced gene activity of the senescent cell protein marker p16 in the guts of aged individuals, indicative of reduced senescent cell numbers. Endurance exercise performed over the course of years alleviated gene activity of a key inflammatory protein – interleukin-6 – associated with senescent cells. Fontana and colleagues went on to show that elevated p16 levels are linked to higher triglyceride to HDL levels – an indicator of increased colon polyp and cardiovascular dysfunction risks. These findings elucidate that intensive endurance exercise likely confers health benefits to the gut by reducing senescent cell abundance in the colon.
Intensive Endurance Exercise Reduces Senescent Cells in Aged Runners’ Colons
Since senescent cells are linked to aging, Fontana and colleagues tested endurance exercise’s effects on senescent cell prevalence in the colon. For this purpose, they took colon biopsies from three groups for comparison: a group of young adults, a group of sedentary older adults, and a group of older adults who regularly practiced endurance running. When they measured gene activity for a protein marker for senescent cells, p16, they found that aged runners and young adults had similar p16 gene activity, but aged, sedentary adults had significantly elevated p16 gene activation. These findings suggest that intensive endurance exercise minimizes the accumulation of senescent cells as marked by gene activity for the protein p16.
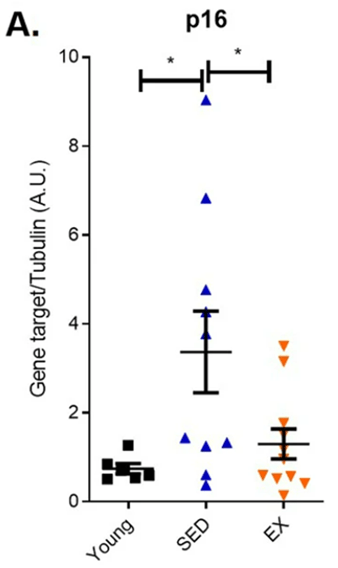
(Demaria et al., 2023 | NPJ Aging) Endurance exercise reduces gene activity (mRNA) levels of a senescent cell marker protein, p16, in aged runners. Compared to young adults (Young), sedentary older adults (SED) displayed higher p16 gene activity, yet endurance exercise curtailed this effect in aged runners (EX).
Since senescent cells emit inflammatory proteins, the Washington University-based researchers looked at how endurance running affects gene activity of a key inflammatory protein, interleukin-6. They found that aged runners had significantly less interleukin-6 gene activity levels than aged, sedentary adults. These results add further support that endurance running reduces the abundance of senescent cells in the colon since low-grade inflammation goes along with senescent cell accumulation.
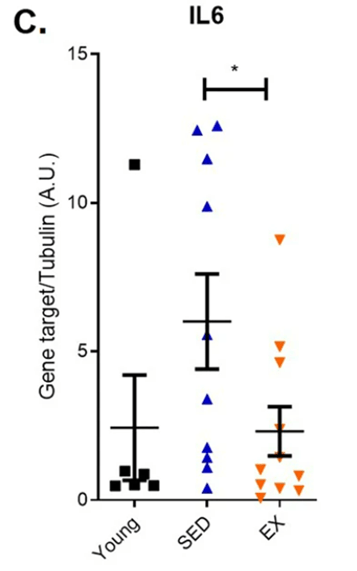
(Demaria et al., 2023 | NPJ Aging) Endurance exercise curtails elevated inflammatory protein interleukin-6 (IL6) in aged runners. Endurance exercise mitigated elevated inflammatory protein interleukin-6 gene activity in aged runners (EX) compared to sedentary older adults (SED).
To find whether senescent cell accumulation plays a role in colon polyp development and potential cardiovascular dysfunction, Fontana and colleagues measured triglyceride to HDL ratios. Researchers have linked elevated triglyceride to high-density lipoprotein ratios to increased risks for colon polyps, metabolic dysfunction, and cardiovascular problems previously. Interestingly, when all of the study participants were pooled, their colon biopsies revealed that elevated p16 protein levels correlate with higher triglyceride to HDL ratios. These findings suggest that elevated p16 levels may induce an increased risk of polyps – 10% of which have the potential to become cancerous – and that endurance running can counter this risk by lowering p16. Moreover, by reducing p16 gene activity, endurance running may also lower the risks for metabolic syndrome and cardiovascular dysfunction.
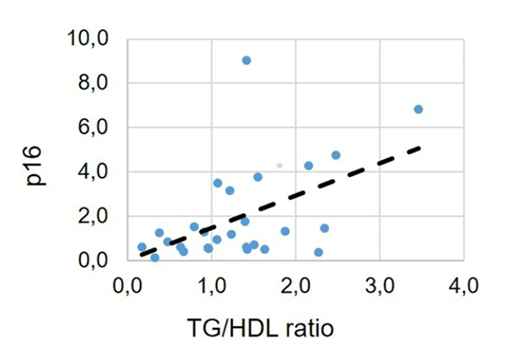
(Demaria et al., 2023 | NPJ Aging) Higher senescent cell marker protein p16 levels were associated with elevated triglyceride to high-density lipoprotein levels that indicate colon polyp, metabolic dysfunction, and cardiovascular problem risks. In pooled aged study participants, elevated p16 senescent cell protein marker levels correlated with higher triglyceride to high-density lipoprotein levels, indicative of higher risks for colon polyp risk – 10% of which have the potential to turn to cancer.
Finding What Exercise Regimens Reduce Senescent Cells and In What Organs
The study has a number of limitations. For example, Fontana and colleagues only examined endurance exercise’s effects on the colon of aged runners. Future studies should examine whether intensive endurance exercise reduces senescent cell buildup in other tissues, such as the liver or kidneys. Another limitation to the study was that high-intensity running regimens of 40+ miles run per week were examined. Not many adults have the time or physical capacity to run this amount during a week. As such, figuring what exercise regimens are most conducive to exercise’s senolytic effects should be explored. Along those lines, a recent study showed that high intensity training lowers p16 gene activity, yet moderate exercise doesn’t. Another study showed that resistance training on a weight machine lowered p16 levels in aged, overweight women.
As future research unfolds, we can expect to learn more and more about how exercise benefits us physically and leads to healthier aging. As such, it would be interesting to find how different exercise regimens compare to senolytic agents regarding their capabilities to eliminate senescent cells.
Model and Dosage
Model: Aged humans (average age of 57 years)
Dosage: Running an average of 48 miles per week
TAGS
Colon, Endurance Exercise, Inflammation, Running, SASP, Senescent Cells
Story Source
Demaria M, Bertozzi B, Veronese N, Spelta F, Cava E, Tosti V, Piccio L, Early DS, Fontana L. Long-term intensive endurance exercise training is associated to reduced markers of cellular senescence in the colon mucosa of older adults. NPJ Aging. 2023 Feb 27;9(1):3. doi: 10.1038/s41514-023-00100-w. PMID: 36849522; PMCID: PMC9971019.
By Victor Ciardha. https://www.nmn.com/news/chinese-study-shows-nmn-enhances-new-potential-therapy-for-heart-attack-damage
Vesicles secreted from NMN-treated stem cells improve heart function while promoting blood vessel formation and reducing tissue damage after myocardial infarction (heart attack) in rats.
Heart tissue damage and scarring (blue) after heart attack (left) is reduced by vesicles secreted from NMN-conditioned stem cells (right).
Highlights:
Heart attacks account for 80% of deaths resulting from heart disease, the leading cause of death worldwide. Heart attacks are caused by cell oxygen deprivation due to blood vessel blockage, leading to fatal levels of heart tissue damage. In an effort to prevent death, it is critical to restore blood flow and repair this tissue damage as soon as possible. Current options include surgery or medications like aspirin.
Now, Pu and colleagues from The First Affiliated Hospital of Nanjing Medical University in China report in Stem Cell Reviews and Reports that vesicles secreted from stem cells treated with NMN (N-Vs) may reduce heart damage and dysfunction after heart attack. Namely, in a heart attack rat model, N-V injections improve heart function, increase blood vessel formation, and reduce tissue damage. These results suggest that stem cell-derived vesicles, especially when conditioned with NMN, can reduce heart damage from heart attack.
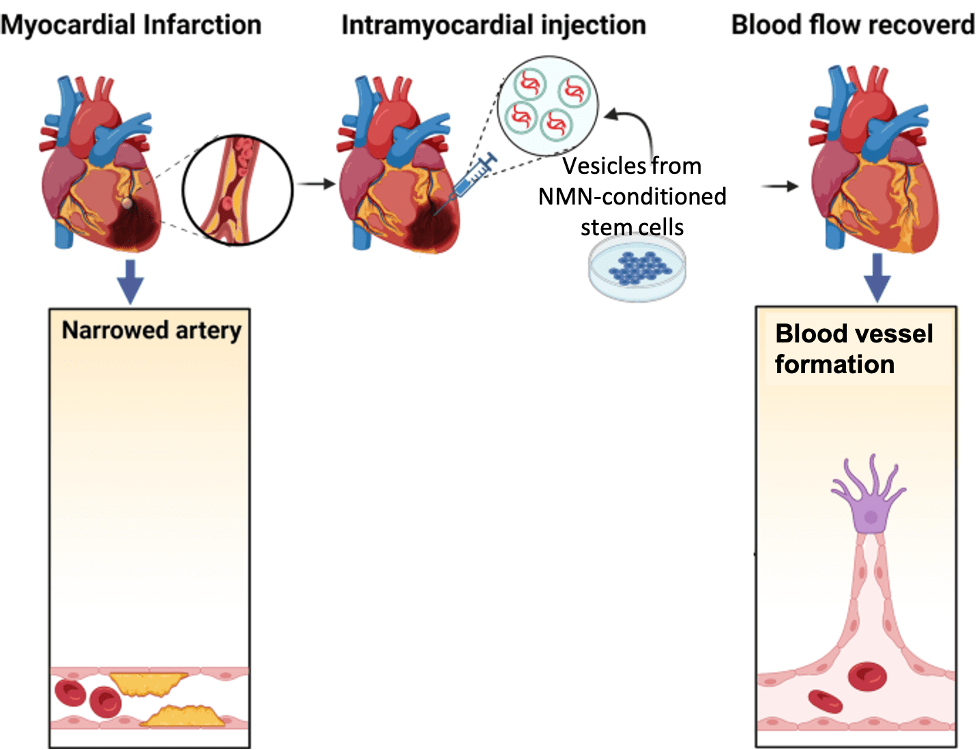
(Pu et al., 2023 | SCRR) Graphical Summary. Myocardial infarction (heart attack) is induced by artery blockage. Injecting damaged heart tissue with vesicles from stem cells treated with NMN reduces heart tissue damage and increases blood vessel formation.
NMN Enhances Stem Cell Vesicle Heart Repair
Recent studies have indicated that stem cells (mesenchymal stem cells) secrete membrane-bound sacs called extracellular vesicles that are filled with potentially therapeutic molecules. Like stem cells, these vesicles possess curative properties. They also have advantages over stem cells, including a lack of immune system rejection and tumor induction. When conditioned with various stimuli, stem cell vesicle content can change, influencing their biological effect.
Pu and colleagues surgically induced coronary artery blockage in rats to cause heart tissue damage and simulate a heart attack. They then injected the rats at the border of the tissue damage with untreated stem cell vesicles or NMN-treated stem cell vesicles. After four weeks, they found that untreated vesicles increased left ventricular ejection fraction — the fraction of blood pumped by the heart. Moreover, N-Vs increased the ejection fraction further, indicating enhancement of heart function repair.
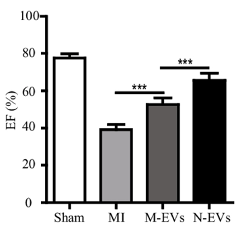
(Pu et al., 2023 | SCRR) NMN Conditioning Improves Stem Cell Vesicle-Mediated Heart Function Recovery. Following heart attack (MI), the ejection fraction (EF) % of the left ventricle is lower than normal (Sham). Vesicles from stem cells (M-EVs) increase the ejection fraction, indicating improved heart function. The ejection fraction is further increased when stem cells are first conditioned with NMN (N-EVs).
The pumping-action of the heart is most efficient when blood flow, mediated by blood vessels, is optimal. Pu and colleagues found that untreated stem cell vesicles increased small artery and capillary density in the heart tissue of rats following heart attack. Additionally, artery and capillary blood vessel density were further increased by N-Vs. These findings demonstrate that NMN enhances vesicle-mediated blood vessel formation, which should increase blood flow.
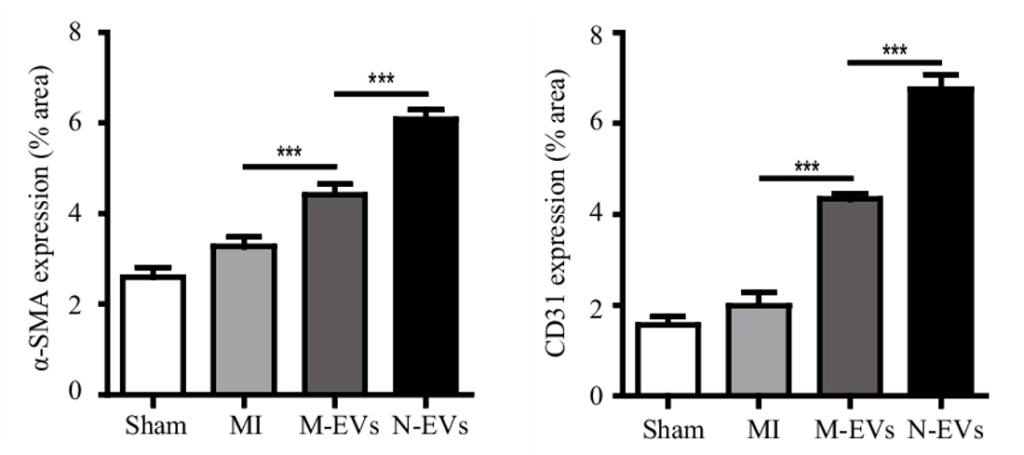
(Pu et al., 2023 | SCRR) NMN Conditioning Improves Stem Cell Vesicle-Mediated Blood Vessel Formation. Compared to normal (Sham), heart attack (MI) insignificantly increases small artery (identified with α-SMA expression, left) and capillary (identified with CD31 expression right) formation to promote tissue recovery. Vesicles from stem cells (M-EVs) significantly (***) increase blood vessel formation, which is further enhanced when stem cells are first conditioned with NMN (N-EVs).
New blood vessel formation contributes to reduced tissue scarring —fibrosis — and cell death in cardiac tissue damaged by heart attack. Pu and colleagues found that fibrosis decreased in response to untreated stem cell vesicles, and that fibrosis decreased more with N-Vs. Furthermore, programmed cell death — apoptosis — was reduced by normal vesicle injections and again reduced by N-V exposure. These findings indicate that NMN enhances stem cell vesicle-mediated cardiac tissue repair.
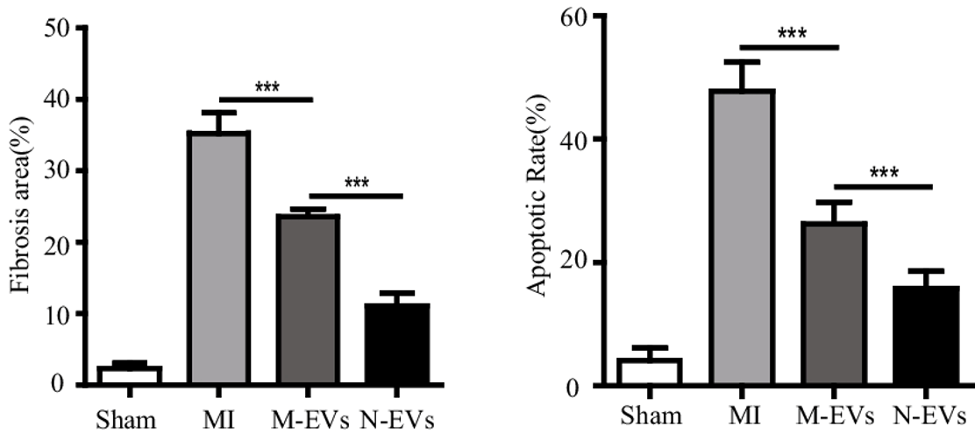
(Pu et al., 2023 | SCRR) NMN Conditioning Improves Stem Cell Vesicle-Mediated Heart Tissue Recovery. Following heart attack (MI), heart scar tissue size (Fibrosis area) % and heart cell death rate (Apoptosis Rate) % markedly increase relative to normal (Sham). Vesicles from stem cells (M-EVs) improve heart tissue remodeling, which is further enhanced when stem cells are first conditioned with NMN (N-EVs).
“Our study demonstrated that extracellular vesicles derived from [stem cells] could improve cardiac function by promoting [blood vessel formation], promoting proliferation, inhibiting apoptosis, reducing fibrosis, and improving cardiac function, which is further enhanced after NMN treatment,” say the authors.
A Future of NAD+-Enhanced Cell-Free Therapy
Stem cells have been applied to the treatment of many diseases, including neurological disease, respiratory disease, fracture healing, and cardiovascular disease. As increasing evidence shows that stem cell vesicles have similar effects, as well as advantages, we may see a shift in research towards so-called cell-free therapy, which is essentially stem cell therapy without the stem cells. Indeed, cell-free stem cell vesicles have been shown to alleviate arthritis and other age-related effects in rodents.
Pu and colleagues take cell-free therapy a step further by showing that NMN, an NAD+ booster, can enhance the therapeutic effects of stem cell-derived vesicles. Previous studies have shown that NMN rejuvenates stem cells, which could explain its enhancement effects. If cell-free therapy proves to be viable, it’s possible that NAD+ boosters or other stem cell rejuvenating drugs could promote the fitness of stem cells and their vesicles to enhance said therapy.
Story Source
Pu, Y., Li, C., Qi, X. et al. Extracellular Vesicles from NMN Preconditioned Mesenchymal Stem Cells Ameliorated Myocardial Infarction via miR-210-3p Promoted Angiogenesis. Stem Cell Rev and Rep (2023). https://doi.org/10.1007/s12015-022-10499-6
Scientists show that phthalate — a chemical used in toys, food packaging, and shampoo — induces features of oocyte aging that can be reversed by NMN.
When oocytes divide, spindles (green) normally attach to chromosomes (red) in an orderly fashion (left). However, phthalate causes spindle disorganization (right), which could lead to infertility.
By Victor Ciardha https://www.nmn.com/news/study-suggests-chemical-found-ubiquitously-in-consumer-products-induces-features-of-reproductive-aging-which-nmn-prevents
Highlights:
The female reproductive system ages faster than any other system in the body, and the ability of oocytes (eggs) to become fertilized declines with age. Thus, accelerating the age of oocytes can promote infertility. Now, a new study from Nanjing Medical University in China suggests that phthalates may accelerate oocyte aging, which could be reversed by NMN.
As reported in Cell Proliferation, Jiang and colleagues show that benzyl butyl phthalate (BBP) induces many common features of aging in mouse oocytes, including reduced fertilization rate. They then show that NMN prevents many of the underlying cellular features of phthalate exposure and aging, including DNA damage, mitochondrial damage, and cell death.
“These findings revealed a mechanism of BBP-induced toxicity on female reproduction and showed that NMN provides an effective treatment for BBP actions,” state Jiang and colleagues.
Chemical in Plastics Damages Oocytes
BBP is a chemical known as a plasticizer, which makes plastics more flexible. BBP is currently undergoing risk evaluation by the Environmental Protection Agency (EPA) and is regarded as an environmental pollutant because of its estrogenic activity. To determine the effects of BBP on female reproduction, Jiang and colleagues fed female mice 1.5 mg/kg of BBP for 8 days and compared them to female mice not exposed to BBP.
To test fertilization rate, the female mice were mated with male mice and their oocytes were removed for examination. While most of the oocytes from the mice not exposed to BBP developed into two-cell embryos, many of the oocytes from BBP-exposed mice went unfertilized. A lack of fertilized eggs suggests higher levels of infertility, the chances of which increase with age.
Aneuploidy — an abnormal number of chromosomes — is the leading cause of miscarriages and developmental disabilities. Aneuploidy is considered a hallmark of oocyte aging, because it increases by 50% with advanced age. Another feature of aging oocytes that leads to aneuploidy is poor spindle — proteins that help equally divide chromosomes — organization. Jiang and colleagues found that oocytes from BBP-exposed mice had both increased spindle disorganization and aneuploidy.
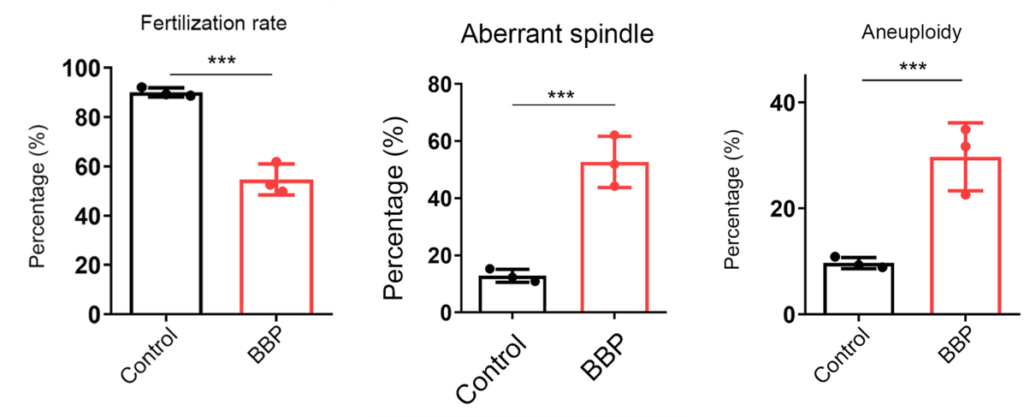
(Jiang et al., 2023 | Cell Proliferation) Phthalate Induces Oocyte Aging. Exposing mouse oocytes to benzyl butyl phthalate (BBP) induces common features of aging, including reduced fertilization rate, aberrant spindle formation, and aneuploidy when compared to normal oocytes (Control).
Cellular aging encompasses universal features that affect not only oocytes but all cells. These features include mitochondrial dysfunction. Mitochondria, like batteries, need to remain at a specific voltage to produce cellular energy. When mitochondria become unhealthy, as with aging, this voltage dissipates. Jiang and colleagues found that the mitochondria contained within the oocytes of BBP-exposed mice had reduced voltages, indicative of poor mitochondrial health. However, this reduction in voltage was reversed by injecting the mice with 200 mg/kg/day of NMN.
Unhealthy mitochondria produce excessive levels of reactive oxygen species (ROS). ROS react with proteins, fats, and DNA, causing damage to cells, a process called oxidative stress. Like mitochondrial dysfunction, oxidative stress is a universal hallmark of cellular aging. Jiang and colleagues found that oocytes from BBP-exposed mice had increased ROS levels, which were reduced to normal levels by NMN.
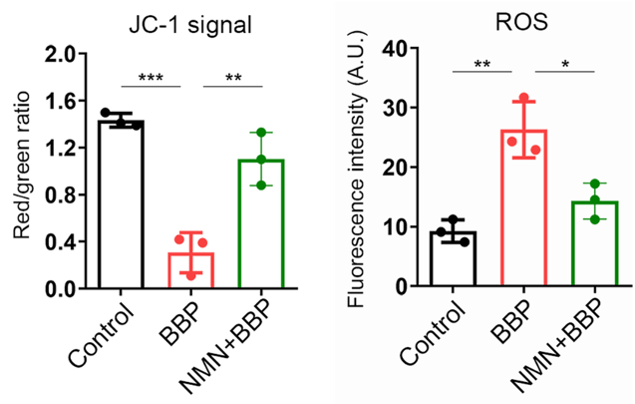
(Jiang et al., 2023 | Cell Proliferation) NMN Restores Mitochondrial Health. Compared to normal mouse oocytes (Control), oocytes exposed to benzyl butyl phthalate (BBP) display reduced mitochondrial health (JC-1 signal) and increased reactive oxygen species (ROS), cell-damaging molecules generated by unhealthy mitochondria. However, NMN prevents this mitochondrial dysfunction and ROS overproduction.
With aging, oxidative stress causes DNA damage that accumulates and leads to further decrements that exacerbate aging. Furthermore, when the voltage of damaged mitochondria reaches a specific threshold, cell death is activated. Jiang and colleagues found that both cell death and DNA damage were increased in the oocytes of BBP-exposed mice. Moreover, NMN prevented the occurrence of both of these aging features.
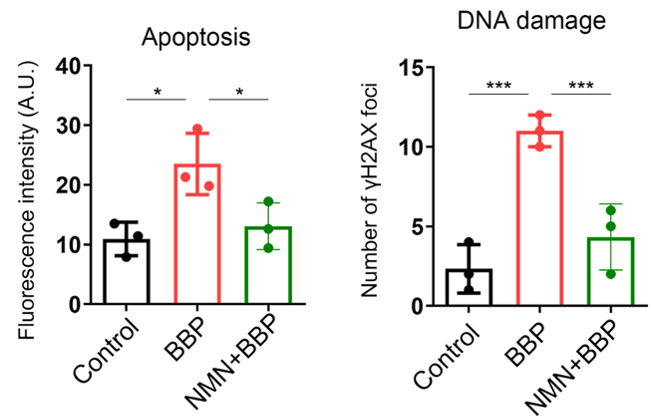
(Jiang et al., 2023 | Cell Proliferation) NMN Restores Cell Health. Compared to normal mouse oocytes (Control), oocytes exposed to benzyl butyl phthalate (BBP) display increased cell death (Apoptosis) and DNA damage. However, NMN prevents this cellular catastrophe.
Phthalates, Human Sperm and Muscle Aging, and How to Avoid Them
Recent studies have linked phthalates to male sperm aging and sarcopenia — the age-related loss of muscle mass and strength — in humans. Studies like these add to concerns of phthalate’s effects on our health, especially reproductive health. To address this concern, a recent study has outlined lifestyle interventions for reducing exposure to phthalate, as well as phenol, another endocrine-disrupting chemical found in many consumer products. One of the interventions is to replace items known to be a source of phthalate or phenol with other products. While knowing which products contain these chemicals may require investigation, the study provides common routes of exposure:
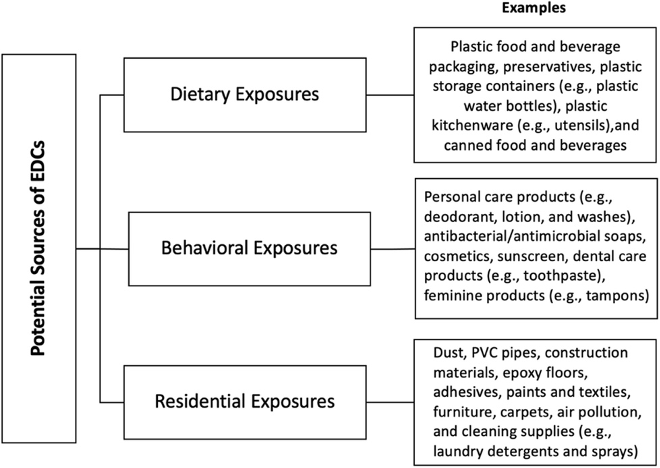
(Martin et al., 2022 | Environment International) Potential Sources of Endocrine-Disrupting Chemicals (EDCs) – phthalates and phenols.
While it is difficult to assess the level of BBP exposure for each individual, some foods have been found to have high levels of BBP, such as vegetable oil with 1.69 mg/kg in one study reported from China. A European study showed that minced meat has 0.078 mg/kg of BBP. Since Jiang and colleagues used 1.5 mg/kg of BBP in this study, these levels are above or near dangerous levels of BBP exposure.
Model and Dosage
Model: Female ICR mice
Dosage: 200 mg/kg/day of NMN intraperitoneally injected and 1.5 mg/kg/day of BBP by force feeding for 8 days
Story Source
Jiang Y, Wang D, Zhang C, Jiao Y, Pu Y, Cheng R, Li C, Chen Y. Nicotinamide mononucleotide restores oxidative stress-related apoptosis of oocyte exposed to benzyl butyl phthalate in mice. Cell Prolif. 2023 Feb 9:e13419. doi: 10.1111/cpr.13419. Epub ahead of print. PMID: 36756972.
Journal Reference
Moghadam ARE, Moghadam MT, Hemadi M, Saki G. Oocyte quality and aging. JBRA Assist Reprod. 2022 Jan 17;26(1):105-122. doi: 10.5935/1518-0557.20210026. PMID: 34338482; PMCID: PMC8769179.
Igarashi H, Takahashi T, Nagase S. Oocyte aging underlies female reproductive aging: biological mechanisms and therapeutic strategies. Reprod Med Biol. 2015 May 9;14(4):159-169. doi: 10.1007/s12522-015-0209-5. PMID: 29259413; PMCID: PMC5715832.
Wasielak-Politowska M, Kordowitzki P. Chromosome Segregation in the Oocyte: What Goes Wrong during Aging. Int J Mol Sci. 2022 Mar 7;23(5):2880. doi: 10.3390/ijms23052880. PMID: 35270022; PMCID: PMC8911062.
Nicotinamide mononucleotide (NMN) alleviates fat tissue inflammation and scarring mediated by low tissue oxygen (hypoxia) – similar to obesity conditions – in mice.
By Brett J. Weiss https://www.nmn.com/news/nmn-attenuates-fat-tissue-inflammation-and-scarring-china-study-suggests
Highlights
Low-grade inflammation driving metabolic conditions like type 2 diabetes and cardiovascular diseases is a key symptom of obesity. This state of inflammation triggers fat tissue fibrosis that volumetrically expands more than normally-functioning fat tissue, leading to oxygen deprivation (hypoxia) from lack of blood flow.
Hypoxia drives further inflammation and fat tissue fibrosis, creating a snowball effect where defective fat tissue perpetuates more damaged and harmful fat tissue. Finding a way to counter this spread of fat tissue damage has become paramount in researchers’ quest to mitigate the obesity epidemic.
Published in Frontiers in Endocrinology, Liu and colleagues from Central South University in China show that NMN injections counter fibrosis in mice with hypoxia-induced fat tissue fibrosis. The researchers show that NMN reduces levels of inflammatory proteins and increases the abundance of pro-insulin sensitivity and anti-inflammatory protein adiponectin. They go on to show that hypoxia increases levels of a protein that initiates fat tissue inflammation and fibrosis – HIF-1ɑ – but that NMN attenuates its abundance.
Findings from the study suggest NMN can ward off the snowball effect where fibrotic fat tissue begets more dysfunctional fat tissue in obesity.
NMN Attenuates Fat Tissue Fibrosis by Reducing HIF-1ɑ Protein Levels
To induce the dysfunctional inflammatory and fibrotic state of fat tissue, Liu and colleagues placed mice in a chamber with low oxygen – hypoxia – for four weeks. They found that hypoxia increased fibrosis about four-fold as measured by the quantity of scar tissue proteins, namely collagen.
NMN treatments more than cut in half the hypoxia-driven fibrosis in fat tissue, suggesting that NMN may provide a means to treat fat tissue fibrosis and dysfunction.
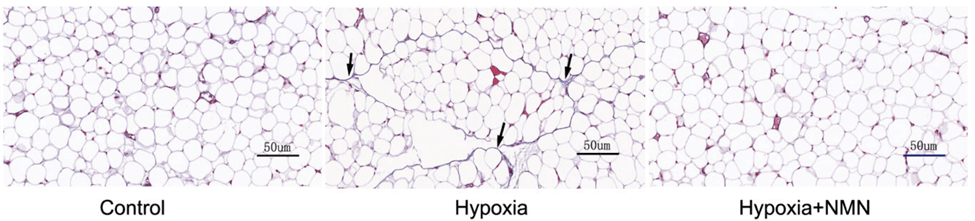
(Wu et al., 2023 | Frontiers in Endocrinology) NMN alleviates fat tissue fibrosis under hypoxic conditions. Fat tissue accumulates fibrous collagen proteins (blue coloration with arrows pointing) that makes up scar tissue and leads to fibrosis under hypoxia (hypoxia column) compared to healthy mice (Control column).
NMN reduces collagen accumulation under hypoxia (Hypoxia+NMN column). Red coloration= cytoplasm within cells.
Since inflammation is one of the factors contributing to fibrosis, the China-based research team tested whether NMN reduces hypoxia-induced fibrosis by lowering inflammatory proteins and increasing anti-inflammatory proteins. Along those lines, they measured levels of the inflammatory molecules TGF-𝛃 and IL-6, along with the pro-insulin sensitivity and anti-inflammatory protein adiponectin.
As expected hypoxia more than doubled levels of the two inflammatory proteins and more than cut in half adiponectin. However, NMN substantially reduced the hypoxia-induced inflammatory protein elevations and significantly increased adiponectin levels after hypoxia.
These results suggest that NMN attenuates hypoxia-driven fibrosis by reducing inflammatory proteins and increasing adiponectin.
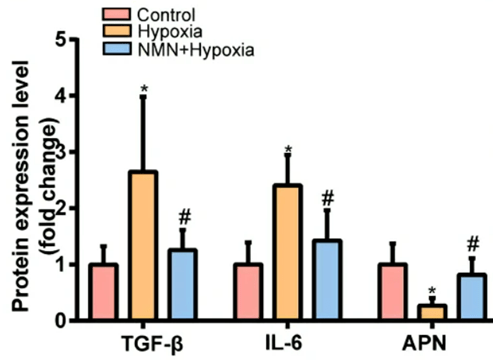
(Wu et al., 2023 | Frontiers in Endocrinology) NMN attenuates inflammatory protein buildup under hypoxia in mouse fat tissue. The inflammatory proteins TGF-𝛃 and IL-6 (three bars on the left and three bars in the middle, respectively) more than double under hypoxia in fat tissue (yellow bars) compared to healthy mice (red bars), but NMN reduces their accumulation (blue bars). The anti-inflammatory and pro-insulin sensitivity protein adiponectin (APN; three bars on the right) shows reduced levels with hypoxia (yellow bar) compared to healthy mice (red bar), but NMN raises its levels toward that of healthy mice (blue bar).
Since the protein HIF-1ɑ initiates and drives hypoxia-induced fat tissue fibrosis, Liu and colleagues measured the protein’s levels with hypoxia. Hypoxia more than doubled HIF-1ɑ levels, but NMN treatments countered the elevated HIF-1ɑ proteins. These findings suggest that NMN reduces HIF-1ɑ levels under hypoxic conditions to reduce inflammation and fibrosis.
“Our study showed that NMN inhibited HIF-1ɑ activation-induced adipose tissue fibrosis and inflammation,” said Liu and colleagues.
Potential New Way to Treat Obesity
With 41.9% of the US population being obese, new treatment strategies to counter fat tissue inflammation, fibrosis, and its subsequent dysfunctional proliferation are paramount. Previous research has shown that NMN counters kidney, liver, and cardiac fibrosis, yet this is the first study to suggest that it can attenuate fat tissue fibrosis.
With recent research from Harvard showing that NMN helps with weight loss, this study illustrates one of the ways that NMN could confer its anti-obesity effects. The study suggests that by inhibiting HIF-1ɑ protein buildup, NMN can thwart fat tissue inflammation and fibrosis, thus inhibiting the perpetual accumulation of dysfunctional fat tissue.
Model and Dosage
Model: C57/B6 mice
Dosage: 500 mg/kg injected intraperitoneally every three days up to five days before hypoxia and then once after hypoxia
TAGS
Adiponectin, Fat, Hypoxia, Inflammation, Nicotinamide Mononucleotide, Obesity, NMN
Story Source
Wu K, Li B, Ma Y, Tu T, Lin Q, Zhu J, Zhou Y, Liu N, Liu Q. Nicotinamide mononucleotide attenuates HIF-1α activation and fibrosis in hypoxic adipose tissue via NAD+/SIRT1 axis. Front Endocrinol (Lausanne). 2023 Jan 26;14:1099134. doi: 10.3389/fendo.2023.1099134. PMID: 36777361; PMCID: PMC9909340.
Treating immune cells that target hepatitis B with nicotinamide mononucleotide (NMN) restores their DNA repair mechanisms and antiviral function.
By Brett J. Weiss https://www.nmn.com/news/new-european-study-restoring-nad-with-nmn-rescues-immune-cell-function-in-hepatitis-b-patients
Highlights
Hepatitis B typically resolves within six months for most people infected, but chronic hepatitis B that doesn’t go away can occur due to CD8 T cell exhaustion. CD8 T cell exhaustion results from high levels of exposure to a pathogen like the hepatitis B virus, triggering T cell DNA damage and a dysfunctional antiviral response. Identifying the cellular processes behind CD8 T cell exhaustion can help with developing therapies for chronic hepatitis B. Some researchers have recently proposed that NAD+ depletion underlies T cell exhaustion.
Published in the Journal of Hepatology, Fisicaro and colleagues from the University of Parma in Italy demonstrate that treating CD8 T cells specific for the hepatitis B virus with NMN improves their production of antiviral proteins – cytokines. Exhausted hepatitis B-specific CD8 T cells were found to have high levels of DNA damage along with dysfunctional DNA repair mechanisms. Findings from the study suggest that NMN and/or CD38 inhibitors can be used to revamp exhausted T cells to ward off chronic hepatitis B, along with potentially other viral infections.
Because Fisicaro and colleagues hypothesized that restoring NAD+ levels with NMN could revamp exhausted CD8 T cells, they isolated these immune cells from chronic hepatitis B patients for testing. Upon treatment with NMN, the cells expressed more antiviral cytokines, specifically a 2.7-fold increase for cytokine interferon gamma (IFN-𝛾), suggesting that NMN restores the CD8 T cells’ antiviral properties.
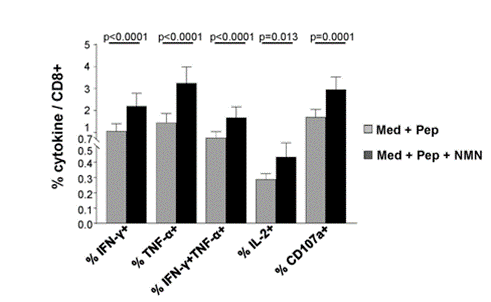
(Montali et al., 2023 | Journal of Hepatology) NMN restores immune cell antiviral cytokine production. Cellular cytokines listed on the X-axis significantly increased following immune cell treatment with NMN and hepatitis B viral proteins (black bars) compared to those treated with hepatitis B viral proteins alone (gray bars). Med + Pep= Cell culture medium with viral peptide stimulation; Med + Pep + NMN= Cell culture medium with viral peptide stimulation and NMN
To examine the cellular damage that goes along with T cell exhaustion in chronic hepatitis B infection, Fisicaro and colleagues compared DNA damage in hepatitis B-specific T cells with influenza (FLU)-specific T cells. The researchers found significantly more DNA damage in the hepatitis B-specific T cells.
They also found that treating the cells with a DNA damage-inducing molecule (etoposide) elicited a trend toward a lower DNA damage response compared to FLU-specific T cells. Since DNA damage responses like the one mediated by PARPs require NAD+, this data suggests that NAD+ deficiency is tied to higher levels of DNA damage in exhausted hepatitis B-specific CD8 T cells.
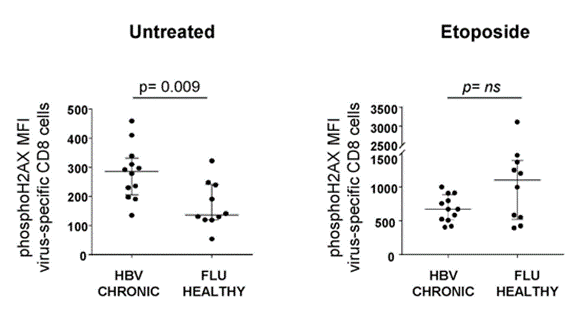
(Montali et al., 2023 | Journal of Hepatology) Hepatitis B-specific CD8 T cells have high levels of DNA damage and a weak DNA damage response to a DNA damaging molecule (etoposide). Left) Compared to FLU-specific CD8 T cells (FLU HEALTHY), those specific for hepatitis B (HBV CHRONIC) exhibit higher levels of markers for DNA damage (phosphoH2AX). Right) A molecule used to induce DNA damage (etoposide) elicits a trend toward lower DNA damage response in hepatitis B-specific immune cells (HBV CHRONIC) compared to FLU-specific immune cells (FLU HEALTHY), suggesting lower NAD+ required for DNA repair.
To explore further how NAD+ repletion with NMN improves exhausted CD8 T cell antiviral cytokine production, Fisicaro and colleagues measured how high levels of the NAD+-consuming CD38 enzyme relate to cytokine levels. They measured one of the primary cytokines – IFN-𝛾 – and found that high CD38 enzyme levels are associated with low IFN-𝛾 levels. These data provide evidence that high CD38 enzyme levels can lead to lower antiviral cytokine production. This also lends credulity to the assertion that low NAD+ levels play an important role in chronic hepatitis B pathology since high CD38 levels would theoretically lower cellular NAD+.
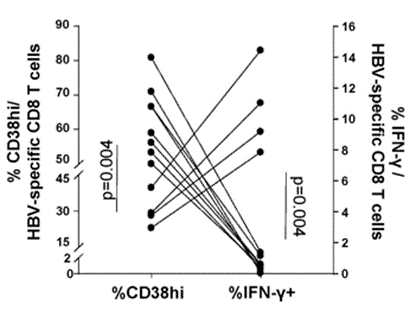
(Montali et al., 2023 | Journal of Hepatology) Higher immune cell CD38 enzyme levels are associated with lower antiviral cytokine levels. H igher percentages of immune cells with elevated CD38 (%CD38hi) are associated with a lower probability of expressing the antiviral cytokine IFN-𝛾 (%IFN-𝛾+).
“Our data show increased DNA damage with limited activation of the DNA repair machinery in [hepatitis B virus]-specific CD8 T cells from [chronic hepatitis B] patients,” say Fisicaro and colleagues. “This strongly suggests that NAD-consuming enzymes, particularly overexpressed CD38, may play a pivotal role in NAD depletion. Reconstitution of many interconnected intracellular functions by NMN supplementation indicates that NAD depletion likely represents an important determinant of T cell exhaustion.”
The study provides evidence that the hepatitis B virus triggers higher CD38 enzyme activation, leading to lower NAD+ levels. Since DNA repair mechanisms like PARPs require NAD+ for their function, this could help explain why exhausted hepatitis B virus-specific T cells have more DNA damage. This could also help explain why DNA repair mechanisms don’t work as well in exhausted hepatitis B-specific CD8 T cells.
Treating CD8 T immune cells with the NAD+ precursor NMN restores antiviral cytokine production, suggesting that NAD+ repletion reconstitutes their function. NMN’s capability to revamp immune cell function provides support that NAD+ depletion contributes to CD8 T cell exhaustion and dysfunction. Whether NMN provides beneficial effects in exhausted CD8 T cells in other types of infections should be explored. If NMN can restore exhausted T cells in other infections, it may be the case that NAD+ makes it easier for immune cells to fight other types of infection, also.
Model
Model: Hepatitis B-specific CD8 T cells from chronic hepatitis B patients
TAGS
Antiviral, Hepatitis, Immunity, Nicotinamide Mononucleotide, T cells
Story Source
Montali I, Berti CC, Morselli M, Acerbi G, Barili V, Pedrazzi G, Montanini B, Boni C, Alfieri A, Pesci M, Loglio A, Degasperi E, Borghi M, Perbellini R, Penna A, Laccabue D, Rossi M, Vecchi A, Tiezzi C, Reverberi V, Boarini C, Abbati G, Massari M, Lampertico P, Missale G, Ferrari C, Fisicaro P. Deregulated intracellular pathways define novel molecular targets for HBV-specific CD8 T cell reconstitution in chronic hepatitis B. J Hepatol. 2023 Mar 7:S0168-8278(23)00167-8. doi: 10.1016/j.jhep.2023.02.035. Epub ahead of print. PMID: 36893853.
NMN prevents chemo-induced mitochondrial defects, such as DNA damage and low cellular energy in neurons derived from human stem cells, a step forward in treating chemo-induced cognitive impairment.
By Victor Ciardha Source: https://www.nmn.com/news/mayo-clinic-scientists-find-nmn-protects-against-chemo-induced-mitochondrial-dysfunction-in-human-neurons
Highlights:
“Chemobrain” is a term used to describe chemotherapy-induced cognitive impairment, which includes impaired memory, attention, and information processing. Up to 60% of chemotherapy patients experience chemobrain years following treatment. Still, there remain no effective treatments due to an unclear understanding of the processes that underlie chemobrain.
Now, researchers from the Mayo Clinic present in Brain Plasticity evidence suggesting NMN can prevent chemobrain by restoring mitochondrial function. In human neurons, Rashid and colleagues show that NMN mitigates cisplatin-induced cellular defects like DNA damage. Furthermore, NMN prevents cisplatin-induced disruptions in mitochondrial function and structure. The findings elucidate the cellular underpinnings of previous findings showing that NMN alleviates chemo-induced memory loss.
Cisplatin is capable of binding to mitochondrial DNA, the DNA found in mitochondria important not only for mitochondrial function but cellular function as well. Cisplatin binding to DNA causes DNA damage and increases the generation of mitochondrial reactive oxygen species (mROS). mROS are highly reactive molecules that, in excessively high concentrations, cause damage to cells — oxidative stress.
To study the effect of NMN on cisplatin-exposed human neurons, Rashid and colleagues took stem cells from a 16-year-old male and chemically transformed them into cortical neurons. The neurons were pretreated with NMN for thirty minutes before being exposed to cisplatin for twenty-four hours. The results showed that with NMN pretreatment, cisplatin-induced DNA damage and increases in mROS were greatly reduced.
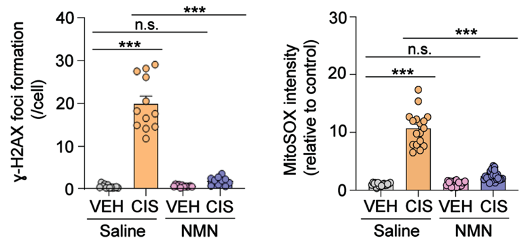
(Rashid et al., 2023 | Brain Plasticity) NMN Reduces DNA Damage and Mitochondrial Reactive Oxygen Species (mROS). In neurons treated with cisplatin (CIS) and saline (orange), DNA damage (𝛾-H2AX foci formation) and mROS (MitoSOX intensity) are greatly increased. However, NMN (blue) largely prevents these cellular defects.
Mitochondria convert oxygen and nutrients into adenosine triphosphate (ATP), a chemical cells use for energy. As impaired mitochondria generate excessive mROS, the resulting oxidative stress dissipates the electrochemical gradient that allows mitochondria to produce ATP, leading to reduced ATP production.
To assess the health of the mitochondrial electrochemical gradient, Rashid and colleagues measured the mitochondrial membrane potential, essentially the voltage of mitochondria. They found that cisplatin reduced both the membrane potential and level of ATP in human neurons. However, this was prevented by NMN pretreatment.
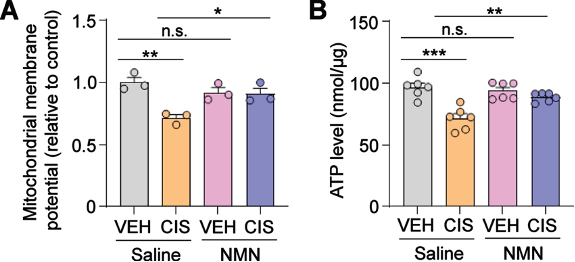
(Rashid et al., 2023 | Brain Plasticity) NMN Prevents Mitochondrial Dysfunction. In neurons treated with cisplatin (CIS) and saline (orange), the mitochondrial membrane potential (A) and ATP levels (B) are reduced. However, NMN (blue) prevents these measures of mitochondrial dysfunction.
A structural feature of mitochondria in response to neural injury is their swelling. Rashid and colleagues measured mitochondrial swelling by assessing the size of mitochondria under a powerful (transmission electron) microscope. Cisplatin caused an increase in mitochondrial area, which was prevented by NMN, suggesting the prevention of swelling.
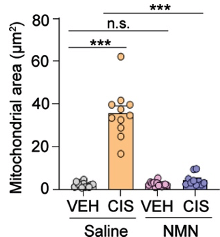
(Rashid et al., 2023 | Brain Plasticity) NMN Prevents Mitochondrial Structural Changes. In neurons treated with cisplatin (CIS) and saline (orange), the mitochondrial area is increased, indicative of mitochondrial swelling and neural injury. However, NMN (blue) prevents this increase in mitochondrial size.
Mitochondrial impairments are prominent features of brain aging and neurodegeneration that underlie cognitive impairment. Thus, the findings of Rashid and colleagues suggest that NMN can potentially aid in preventing brain aging and neurodegeneration, especially in response to chemotherapies like cisplatin.
NMN has also been shown to prevent heart damage in response to doxorubicin, a chemotherapy for cancers like leukemia, and protect against intestinal wall injury in response to radiation therapy, commonly used to treat abdominal and pelvic tumors in older individuals. Thus, with more studies, we may see more evidence showing that NMN can prevent the side effects of various cancer therapies.
Story Source
Rashid MA, Oliveros A, Kim YS, Jang MH. Nicotinamide Mononucleotide Prevents Cisplatin-Induced Mitochondrial Defects in Cortical Neurons Derived from Human Induced Pluripotent Stem Cells. Brain Plast. 2022 Dec 20;8(2):143-152. doi: 10.3233/BPL-220143. PMID: 36721392; PMCID: PMC9837732.