A new study has revealed that restoring NAD+ levels in the aging brain may be possible through the use of nicotinamide mononucleotide (NMN) or small vesicles containing the NAD+ synthesizing enzyme NAMPT (eNampt). The research, conducted by scientists at Washington University, showed that treating aged mice with NMN or eNampt led to increased NAD+ levels in various regions of the hypothalamus, a brain region responsible for maintaining overall health and homeostasis.
The study utilized a novel method to accurately measure NAD+ levels in the small subregions of the hypothalamus, and the results showed that NAD+ levels decline significantly in three of the four measured areas in aged mice. The researchers found that a single shot of 300 mg/kg of NMN was enough to restore NAD+ levels in all three regions. Injecting aged mice with small vesicles from young mice containing NAMPT also resulted in nearly a 50% increase in NAD+ levels in the hypothalamus.
The findings of the study open up the possibility of future research on the effects of reduced NAD+ in the hypothalamus and other brain regions. The results also confirm that NMN is an effective method of replenishing NAD+ levels in the hypothalamus of aged mice, which can help maintain brain health and prevent cognitive decline.
In conclusion, the study highlights the importance of NAD+ in preserving brain health, especially in aging. The new method of measuring NAD+ levels in small tissues and the positive results of NMN supplementation offer hope for the future of brain health and wellness.
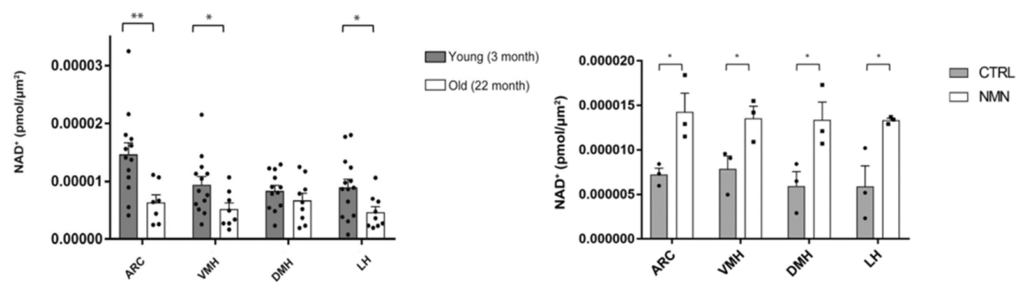
(Imai et al., 2023 | NPJ Aging) NMN attenuates falling NAD+ levels in four subregions of the hypothalamus. (Left) compared to young mice (grey), aged mice (white) show declining NAD+ levels in the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), and lateral hypothalamus (LH) of the hypothalamus. (Right) NMN (white) increases NAD+ levels in all four hypothalamic subregions: ARC, VMH, LH, and dorsomedial hypothalamus (DMH).
NAMPT-Containing Vesicles Boost Hypothalamic NAD+ Levels
NAMPT has been widely recognized as an effective NAD+ booster in various tissues and a promoter of longevity. Previous studies have shown that transplanting small vesicles from young mice into aged mice can increase NAD+ levels and extend lifespan. With this in mind, the researchers, Imai and colleagues, aimed to investigate the effects of similar vesicles on NAD+ levels in the hypothalamus.
The results showed that injecting aged mice with vesicles containing NAMPT from young mice resulted in a substantial increase in NAD+ levels in the hypothalamus. These findings suggest that these vesicles have potent NAD+-enhancing properties in the hypothalamus and raise the possibility that this treatment could reverse age-related changes in sensory processing and emotional control in the hypothalamus.
In conclusion, NAMPT-containing vesicles hold promise as a treatment for boosting NAD+ levels in the hypothalamus and may have potential for alleviating age-related changes in the brain. Further research is needed to fully understand the impact of this treatment on aging and brain health.
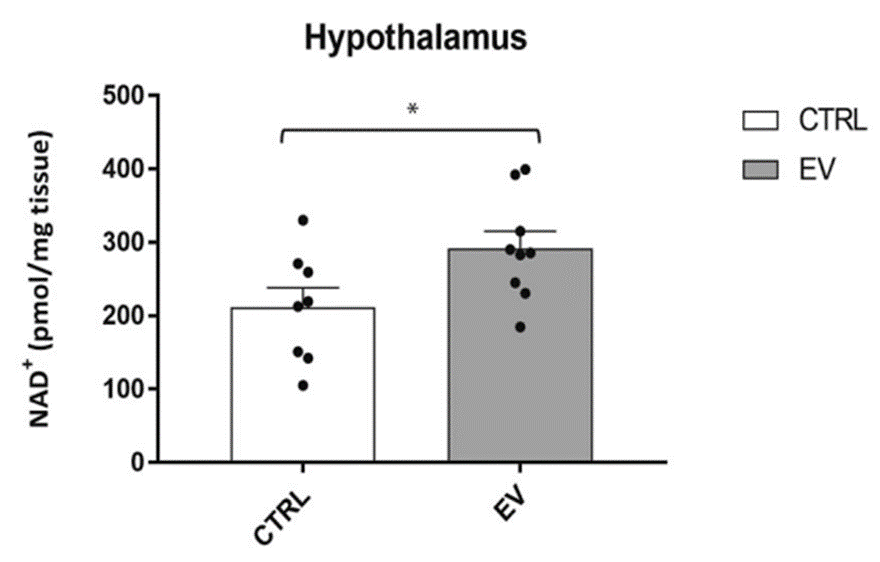
(Imai et al., 2023 | NPJ Aging) NAMPT-containing vesicles raise hypothalamic NAD+ levels. Compared to untreated controls (CTRL), aged mice treated with NAMPT-containing vesicles from young mice (EV) have around 50% more hypothalamic NAD+.
NMN and eNAMPT as Possible Treatments for Brain Aging
Thanks to the new method developed by Imai and colleagues for accurately measuring hypothalamic NAD+ levels, we now have the means to monitor brain aging and potentially discover new therapies. Our brains inevitably suffer a decline in function as we age, due to constant damage from stressors like inflammation and DNA damage, which rely on NAD+ for protection. Therefore, finding ways to increase NAD+ in various tissues is critical to slowing down aging and preserving organ function.
The study results show that NMN and eNAMPT can effectively raise NAD+ levels in the aged hypothalamus, suggesting that these treatments could mitigate the effects of brain aging, such as poor sleep, decreased physical ability, and diminished cognition. However, further research is needed to validate these findings.
Model and Dosage
Model: C57BL/6J mice
Dosage: 300 mg/kg NMN intraperitoneal injection at 22 months of age
Story Source
Johnson, S., Yoshioka, K., Brace, C.S. et al. Quantification of localized NAD+ changes reveals unique specificity of NAD+ regulation in the hypothalamus. npj Aging 9, 1 (2023). https://doi.org/10.1038/s41514-023-00098-1
https://www.nmn.com/news/washington-university-study-shows-nmn-replenishes-nad-in-the-aging-brain
Brazilian scientists show that resveratrol reduces markers of inflammation and senescence (functional deterioration) in brain cells taken from aged rats.
By Victor Ciardha Original Article: www.nmn.com
Highlights:
The hypothalamus controls everything from our reproductive success to our sleep-wake cycles. Scientists hypothesize that when this region of the brain becomes inflamed, our body is knocked out of homeostasis, leading to metabolic diseases like obesity, as well as aging. A new study out of the Federal University of Rio Grande do Sul in Brazil suggests that such inflammation can be reduced by the grape molecule resveratrol.
In the latest issue of Molecular and Cellular Biochemistry, Sovrani and colleagues tested the effects of resveratrol, known for its antioxidant, anti-inflammatory, and anti-aging properties, on cultured brain cells from aged rats. They found that treating these brain cells grown in a dish with resveratrol reduces the activation of genes and proteins associated with inflammation and senescence. Furthermore, resveratrol activated the gene for SIRT1, one of the most promising anti-aging targets.
Resveratrol Reduces Brain Cell Inflammation and Senescence
Astrocytes are constantly mingling with neurons and other central nervous system cells (i.e. glial cells), as well as blood vessels, to make sure extracellular molecules (ions, water, neurotransmitters) are balanced and in order. However, during aging, astrocytes adopt a pro-inflammatory state, potentially leading to neurotoxicity and neuroinflammation.
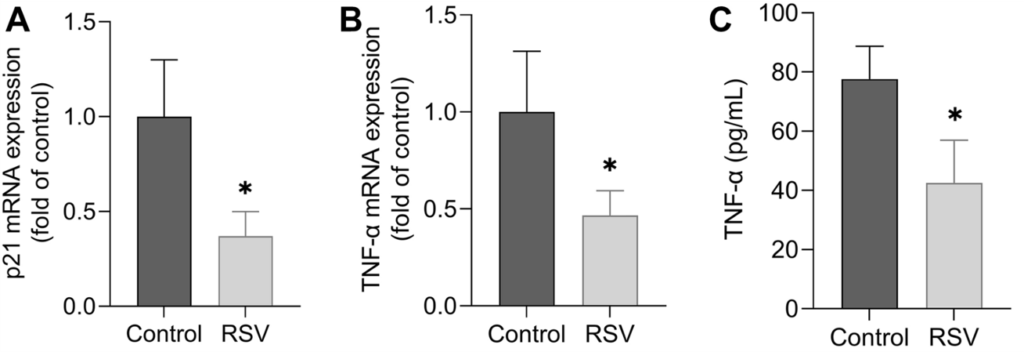
Sovrani and colleagues isolated astrocytes from the hypothalamus of 24-month-old rats (equivalent to 70 human years) and grew (cultured) them in a dish. They then added resveratrol to the dish and measured changes in gene and protein activity. The measurements suggested that, overall, resveratrol reduced inflammation. Additionally, a marker for cell senescence called p21 was reduced, suggesting a reduction in senescence.
(Sovrani et al., 2022 | Mol. Cell. Biochem.) Resveratrol Reduces Senescence and Inflammation. In astrocytes from the hypothalamus of rats, resveratrol (RSV) reduces an mRNA marker for senescence called p21 (A), and an mRNA (B) and protein (C) marker for inflammation called TNF-α.
The past few decades of aging research have brought to light a collection of molecules associated with increased lifespan and ameliorating age-related diseases. Among these molecules are SIRT1 and AMPK – an enzyme that plays key roles in cellular energy homeostasis. These molecules are activated in response to caloric restriction, the most experimentally reproducible intervention for increasing lifespan.
Sovrani and colleagues found that while resveratrol increased SIRT1 gene activation in astrocytes from the hypothalamus of old rats, it decreased AMPK activation. This is of concern because resveratrol has previously been shown to increase AMPK in astrocytes. Thus, more studies will be needed to confirm how resveratrol affects AMPK activation in the astrocytes of the aged hypothalamus.
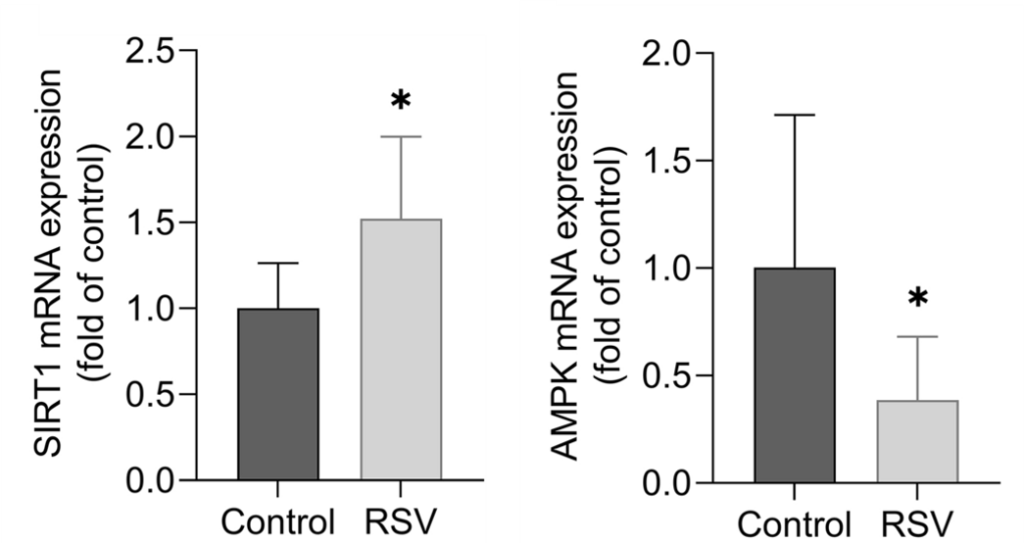
(Sovrani et al., 2022 | Mol. Cell. Biochem.)
Resveratrol Increases SIRT1. In astrocytes from the hypothalamus of rats, resveratrol (RSV) increases SIRT1 mRNA (left) and decreases AMPK mRNA (right).
The Brazilian researchers also looked at genes involved in aging processes other than inflammation, senescence, and longevity. For example, they found that resveratrol activates genes for antioxidant enzymes and others that control mitochondrial health. Additionally, genes for balancing glutamate were increased, suggesting a reduction in glutamate toxicity. Glutamate is a neurotransmitter that can be toxic to the brain if not properly balanced and regulated, which may underlie diseases like Alzheimer’s disease.
Combining Resveratrol with NMN
Resveratrol is a polyphenol (plant molecule) that naturally occurs in grapes, wines, peanuts, and berries, although in small quantities. Resveratrol can also be taken in supplement form at doses associated with its anti-aging benefits. Both resveratrol and NMN have been shown to improve cognition in rats, which may be a result of reduced hypothalamic inflammation. Interestingly, it has been reported that combining NMN and resveratrol reduces NAD+ levels in the brain but increases NAD+ in organs like muscle in mice.
Another curiosity, an NMN transporter in the hypothalamus is associated with slowing muscle decline. Clearly, more research is needed to determine how resveratrol and NMN affect the hypothalamus to then regulate the aging of other organs like muscle.
Source
Sovrani V, Bobermin LD, Santos CL, Brondani M, Gonçalves CA, Leipnitz G, Quincozes-Santos A. Effects of long-term resveratrol treatment in hypothalamic astrocyte cultures from aged rats. Mol Cell Biochem. 2022 Oct 22. doi: 10.1007/s11010-022-04585-z. Epub ahead of print. PMID: 36272012.
As an antioxidant promoter, NMN decreases lung damage in a mouse model of silicosis — an inflammatory lung disease caused by long-term inhalation of silica dust particles.
From left to right: normal, silica-injured, and NMN-treated silica-injured lung tissue stained for scarring (purple).
By Bella Richman
Source: https://www.nmn.com/news/scientists-find-nmn-counters-lung-damage-caused-by-dust-inhalation
Highlights:
Aging is often associated with an increased risk of lung injury and lung disease, and advanced age has been shown to lead to an enhanced inflammatory response to lung assaults. Now, a recent study shows that nicotinamide mononucleotide (NMN) treatment may reduce injury due to silica inhalation, possibly modeling NMN’s ability to help reduce injury due to other inhaled particulates.
The study, out of China, published in Nutrients, focused on male mice subjected to silica-induced lung injury, or silicosis. Silicosis promotes cellular damage — oxidative stress — by increasing reactive oxygen species (ROS) – highly reactive oxygen-containing molecules. Treating mice with NMN reduced lung damage, leading to positive tissue changes and reduced inflammation. Additionally, oxidative stress was decreased, ROS reduced, and glutathione increased.
“This study reveals that NMN supplementation might be a promising strategy for mitigating oxidative stress and inflammation in silicosis,” the scientists wrote.
NMN Reduces Lung Tissue Damage in Silicosis
To model silicosis, silica was introduced directly into the trachea of mice. The mice were then treated with either saline (meaning no treatment), low-dose NMN (500 mg/kg/day), or high-dose NMN (1000 mg/kg/day). After 28 days, both doses of NMN reduced collagen within the lungs – indicative of decreased lung scarring. NMN treatment also maintained the structure of alveoli – air sacs in the lungs where oxygen is exchanged.
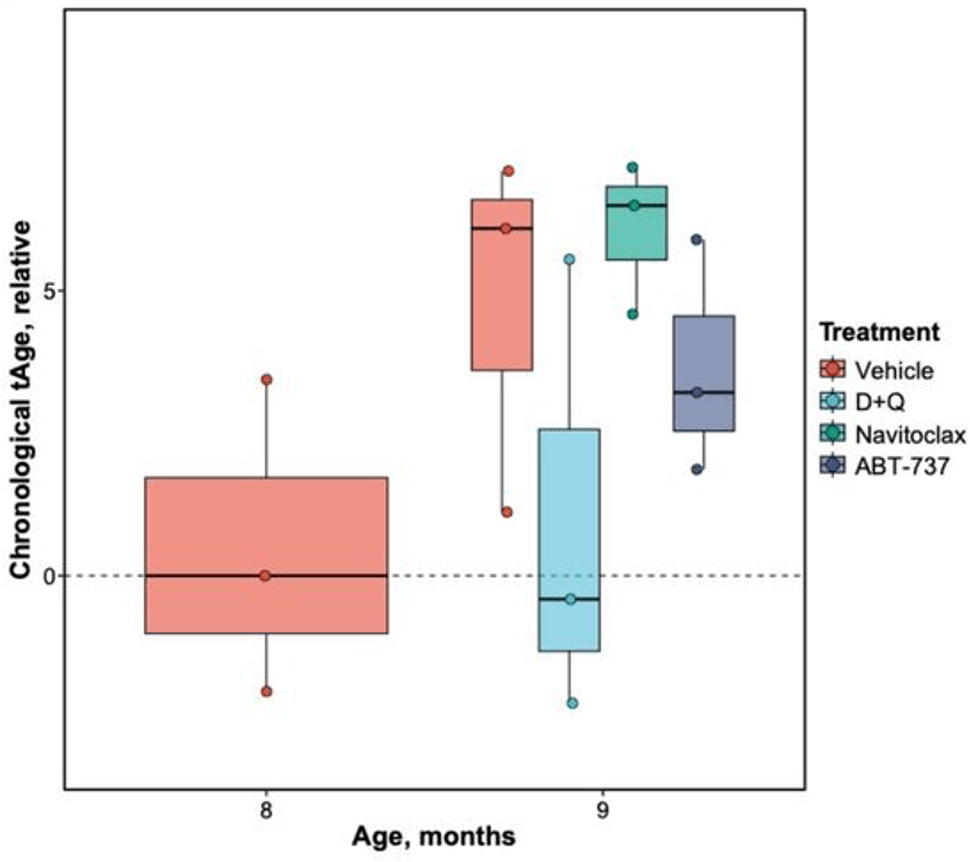
(Wang et al., 2023 | Nutrients) NMN Reduces Lung Injury in Silicosis Model. Compared to normal mice (Sham), mice exposed to silica (Vehicle) display increased lung scarring, as measured by the B) Ashcroft score and C) collagen volume fraction. However, high (NMN-H) and low (NMN-L) dose NMN reduces scarring. NMN-Con = no silica with NMN. #### difference from untreated (Vehicle) mice ** difference between low and high dose NMN.
Silica particles are nearly impossible for the body to degrade, leading to continuous damage to the lungs via oxidative stress. This oxidative stress recruits inflammatory cells to the site of damage, inducing more inflammation. However, the researchers found that with NMN treatment, this cycle of continuous assault was prevented with NMN restoring inflammatory cell levels back to nearly normal levels after 28 days of treatment.
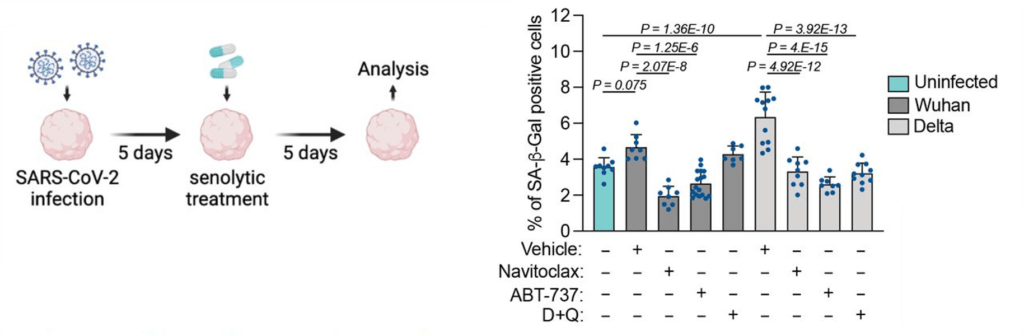
(Wang et al., 2023 | Nutrients) NMN Reduces Infiltrating Inflammatory Cells in Silicosis Model. High (NMN-H) and low (NMN-L) dose NMN reduces the percent of macrophages – infiltrating inflammatory cells – in the lungs of mice exposed to silica (Vehicle), close to levels of mice that did not have silica injuries or NMN treatment (Sham). NMN-Con = no silica with NMN. #### difference from untreated (Vehicle) mice.
Furthermore, Wang and colleagues found that, after 28 days, both high and low doses of NMN effectively decreased ROS. Additionally, glutathione – a key antioxidant that neutralizes ROS – was increased with NMN,suggesting that NMN can help reduce the oxidative stress caused by silica.
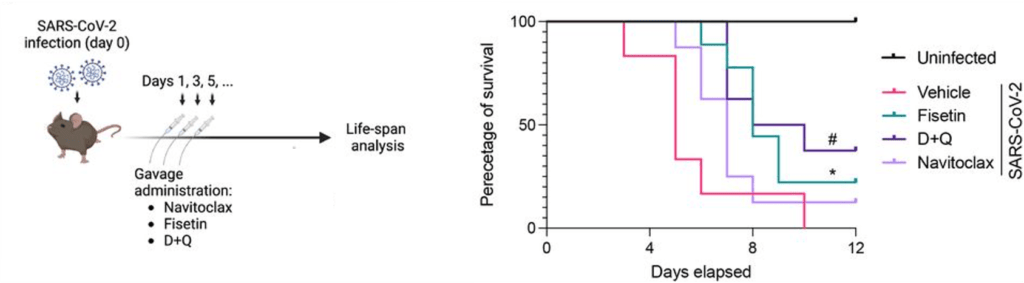
(Wang et al., 2023 | Nutrients) NMN Decreases Reactive Oxygen Species and Increases Glutathione Levels. High (NMN-H) and low (NMN-L) doses of NMN decrease D) ROS (Mean fluorescent intensity) and increase F) glutathione (GSH) levels in the lungs of mice exposed to silica (Vehicle), close to normal mice (Sham). NMN-Con = no silica with NMN. difference from untreated (Vehicle) mice ** difference between low and high dose NMN.
NMN for Lung Injuries
Wang and colleagues show that NMN may be a promising treatment for silicosis. Other models of lung injury, including sepsis-induced lung injuries and cigarette-induced lung fibrosis, have also been shown to be positively affected by NMN treatment. These studies hold promise for both those affected by lung injury, as well as those affected by aging lung tissue. With NMN effectively reducing oxidative stress in the lungs, it holds the possibility of doing the same for aging tissue.
Previous work has actually shown that NMN helps to reduce signs of aging and functional decline in the lungs of old mice. However, clinical research studies with long-term follow-up are needed before determining the full extent of NMN’s benefits on human lungs.
Model and Dosage
Model: Male C57BL/6J mice
Dosage: 500 mg/kg or 1000 mg/kg of NMN via intratracheal instillation
Story Source
Wang L, Zhao M, Qian R, Wang M, Bao Q, Chen X, Du W, Zhang L, Ye T, Xie Y, Zhang B, Peng L, Yao Y. Nicotinamide Mononucleotide Ameliorates Silica-Induced Lung Injury through the Nrf2-Regulated Glutathione Metabolism Pathway in Mice. Nutrients. 2023; 15(1):143. https://doi.org/10.3390/nu15010143
Jilin University researchers show that nicotinamide mononucleotide (NMN) reduces stem cell senescence (aging) and restores mitochondrial function in rats by increasing the activity of sirtuin 3 (Sirt3) — a longevity-associated protein.
Protein Data Bank Image of Sirtuin3
By Vanni Alagona Published: 4:41 p.m. PST Jan 10, 2023 | Updated: 5:37 p.m. PST Jan 10, 2023
Highlights
Stem cells have garnered extreme attention for their potential applications in regenerative medicine. They possess self-renewal properties shown to contribute to tissue healing and cartilage regeneration. However, their regenerative potential is severely compromised by cellular senescence, which occurs in the later stages of stem cell growth. Therefore, finding ways to delay senescence in growing stem cells is vital to sustaining their therapeutic potential.
Now, researchers from Jilin University in China report in the International Journal of Molecular Sciences that nicotinamide mononucleotide (NMN) attenuates stem cell senescence in late-stage stem cells. Wang and colleagues show that treating old stem cells with NMN limits senescent cell burden and boosts mitochondrial function, which is key to delaying senescence. Notably, the investigators demonstrate that NMN’s beneficial effects rely on Sirt3 activation, highlighting a potential mechanism of action.
NMN Boosts Mitochondria and Reduces Senescence in Old Stem Cells
Dysfucnfucntional mitochondria hinder the production of ATP, our cell’s energy currency. Moreover, they exacerbate the production of reactive oxygen species (ROS), harmful compounds that induce oxidative stress. These consequences are known drivers of accelerated aging and have been shown to promote cellular senescence. With this in mind, the investigators examined whether treating old stem cells with NMN could improve mitochondrial function and limit senescent cell burden.
Prior to treatment, the old stem cells exhibited decreased ATP production and increased ROS levels, indicating poor mitochondrial function. Following treatment, the old stem cells had significantly higher ATP production and drastically lower ROS levels. Furthermore, NMN treatment lowered the number of senescent cells in old stem cells. Taken together, the initial findings demonstrate that NMN attenuates cellular senescence by restoring mitochondrial function.
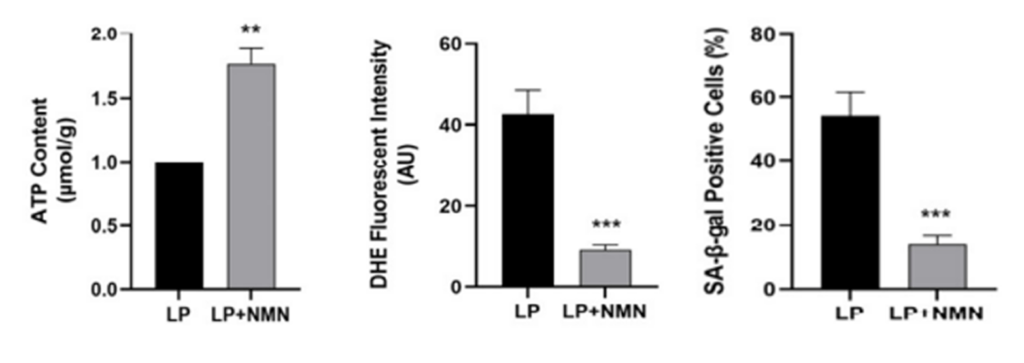
(Wang et al., 2022 | Int. J. Mol. Sci.) NMN increases ATP, reduces ROS, and decreases senescence. Prior to treatment, old stem cells (LP) have lower ATP levels (left), more ROS (middle), and increased senescence (right). However, treating old stem cells with NMN (LP+NMN) restores ATP, lowers ROS, and limits senescence.
NMN’s Beneficial Effects Rely on Sirt3 Activation
Sirt3 is a critical mitochondrial protein that helps regulate oxidative stress and plays a key role in ATP production. Given Sirt3’s involvement in these critical processes, Wang and colleagues tested whether NMN altered Sirt3 activity in old stem cells. Accordingly, Sirt3 activity was significantly higher in old stem cells treated with NMN, highlighting a potential connection between Sirt3 activation and the observed mitochondrial benefits following NMN treatment.
To further elucidate whether Sirt3 activation governed NMN’s mitochondrial benefits, the investigators examined whether inhibiting the Sirt3 protein in old stem cells would reverse NMN’s effects on mitochondria following treatment. The results showed that blocking sirt3 abolished the effects of NMN, suggesting the effects of NMN are mediated by Sirt3.
Overall, the findings highlight a potential mechanism linking Sirt3 activation, healthy mitochondria, and decreased senescence in old stem cells.
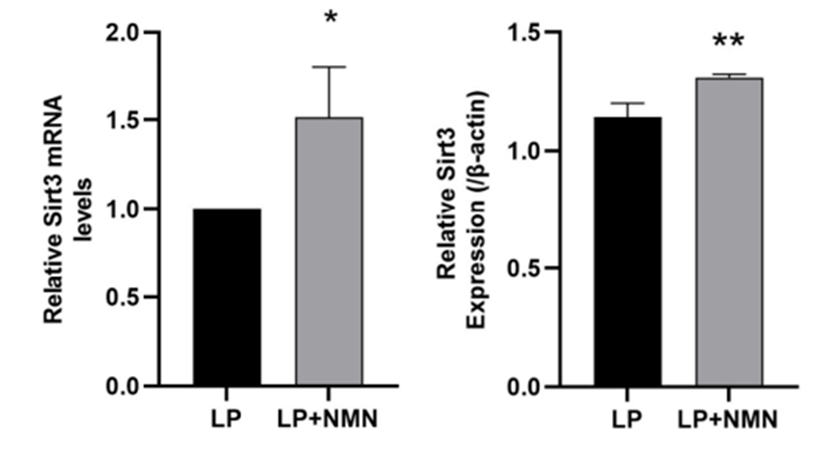
(Wang et al., 2022 | Int. J. Mol. Sci.) NMN increases Sirt3 activity. Sirt3 mRNA (left) and protein (right) levels are lower in untreatred stem cells (LP) than NMN-treated stem cells (LP+NMN).
Sirtuin Activation and Longevity
Studies continue to demonstrate the importance of sirtuins in increasing longevity. Notably, sirtuins are critical to repairing and maintaining the integrity of our genetic blueprints (DNA), which drive the majority of age-related diseases when compromised. However, sirtuins require NAD+ for activation. Thus, NAD+ precursors like NMN are prime candidates to spark sirtuins health-boosting effects. In the present study, NMN’s ability to enhance mitochondrial function and decrease stem cell senescence through Sirt3 regulation demonstrates that NAD+ precursors could restore the therapeutic potential of stem cells and delay aging features by activating sirtuins.
Model and Dosage
Model: Early passage and late passage stem cells derived from health, 1-2 month-old male Wistar rats bone marrow.
Dosage: 100 µM NMN for 48 hours in culture.
Story Source
Wang H, Sun Y, Pi C, Yu X, Gao X, Zhang C, Sun H, Zhang H, Shi Y, He X. Nicotinamide Mononucleotide Supplementation Improves Mitochondrial Dysfunction and Rescues Cellular Senescence by NAD+/Sirt3 Pathway in Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2022; 23(23):14739. https://doi.org/10.3390/ijms232314739
The senolytic fisetin increases the muscle size and strength of prematurely aged mice by increasing muscle stem cells and reducing fat stem cells.
Author, Dr. Paul D. Robbins | University of Minnesota By Victor Ciardha. www.nmn.com
Highlights:
The older we get, the weaker we tend to become, largely due to muscle atrophy (shrinkage) and reduced strength. While weakness can be countered by resistance exercise, with age our muscles become less capable of regenerating or growing. This lack of regenerative capacity is related to impaired muscle stem cells. Now, researchers are getting closer to rejuvenating aged muscle by restoring stem cell function.
Scientists based in the United States and China report in the Journal of Cachexia, Sarcopenia and Muscle that the senolytic fisetin rescues stem cell function in a mouse model for premature aging (progeria). Liu and colleagues show that fisetin, while increasing muscle cell size and strength, decreases the formation of non-living scar tissue in the muscle of progeria mice. Fisetin also decreases senescent fat stem cells and increases muscle stem cells in the muscle of progeria mice. These findings suggest that fisetin can help restore muscle regenerative capacity.
Senolytics Restore Muscle Growth Capacity and Strength
Hutchinson-Gilford progeria syndrome is a genetic disease associated with premature aging (progeria) that causes muscle atrophy. Liu and colleagues fed mice modeling progeria 100 mg/kg of fisetin once per week for 4 weeks. In addition to reducing muscle atrophy, fisetin treatment decreases the formation of scar tissue (fibrosis), which impairs muscle regeneration and is a major cause of muscle weakness. Fisetin also increased the strength of the progeria mice, as measured by how long they could hang from a bar.
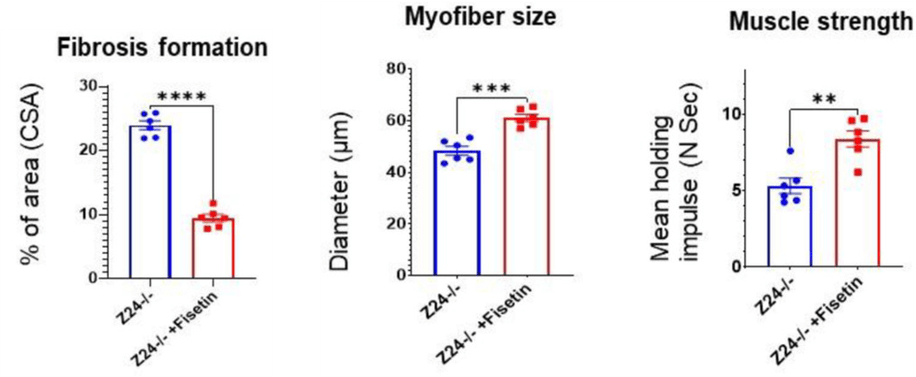
(Liu et al., 2022 | J. Cachexia Sarcopenia Muscle)
Fisetin Reduces Muscle Scarring and Atrophy while Increasing Strength. Fisetin-treated progeria mice (Z24-/- +Fisetin) have reduced muscle scarring (Fibrosis formation), increased muscle cell size (Myofiber size), and increased strength (Muscle Strength) compared to untreated progeria mice (Z24-/-).
Muscle stem cells lose their ability to proliferate and form new muscle cells with age, but the reason for this is unclear. Fibrotic-fat stem cells (fibro-adipogenic progenitors) interact closely with muscle stem cells to facilitate muscle regeneration. However, in disease states, these fat stem cells promote fibrosis and fat deposition. Furthermore, when fat stem cells enter a senescent state, they secrete molecules that impair surrounding cells, including muscle stem cells.
Liu and colleagues found that fisetin treatment eliminated senescent cells in the muscle of progeria mice, as expected from a senolytic. They also found that fisetin reduced the number of fat stem cells, some of which could have been senescent. Furthermore, fisetin increased the number of muscle stem cells, suggesting that their proliferative capacity had been restored. Along with other experiments, these findings suggest that fisetin restores muscle stem cell function by eliminating senescent fat stem cells.
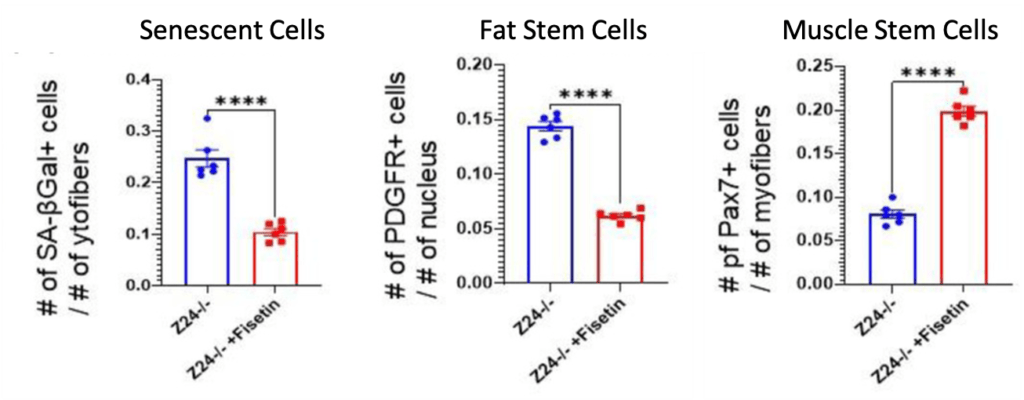
(Liu et al., 2022 | J. Cachexia Sarcopenia Muscle)
Fisetin Reduces Senescent Cells and Fat Stem Cells while Increasing Muscle Stem Cells. Fisetin-treated progeria mice (Z24-/- +Fisetin) have reduced senescent cells (Senescent Cells) and fat stem cells (Fat Stem Cells), and increased muscle stem cells (Muscle Stem Cells) compared to untreated progeria mice (Z24-/-).
Overall, it would seem, from the findings of Liu and colleagues, that fat stem cells could accumulate with aging to increase fibrosis and fat deposition, leading to muscle weakness. These fat stem cells, and probably other muscle resident cells, could become senescent and impair muscle stem cells, leading to diminished muscle regenerative capacity. By eliminating senescent cells, namely senescent fat stem cells, fisetin can restore muscle regeneration, leading to increased muscle growth and strength.
Senolytics May Reverse Muscle Aging & Prolong Lifespan
With senolytics like fisetin on the cutting edge of aging science, human studies measuring their effect on muscle decline have been limited. One study, however, showed that 10 mg/day of the senolytic quercetin was associated with reduced odds of frailty in middle-aged and older adults. The muscle of older adults also has more senescent cells, which has been associated with reduced strength. In mice, the senolytic combo dasatinib plus quercetin D + Q improves muscle regeneration and muscle-building potential in old age. Therefore, senolytics could possibly prevent or reverse signs of muscle aging.
The importance of maintaining muscle mass and strength cannot be understated, as reduced grip strength is associated with an increased risk of mortality. Several studies, including one from the United Kingdom (U.K.) and one from China, have shown an association between reduced grip strength and increased risk of death. Another study revealed that low grip strength is associated with an increased risk of death from various types of cancer. Furthermore, a recent study showed that reduced grip strength is associated with accelerated aging. Thus, maintaining strength may be important for living a long life.
Model and Dosage
Model: Z24-/- mouse model for progeria
Dosage: 100 mg/kg fisetin weekly via oral gavage for 4 weeks
Story Source
Liu L, Yue X, Sun Z, Hambright WS, Wei J, Li Y, Matre P, Cui Y, Wang Z, Rodney G, Huard J, Robbins PD, Mu X. Reduction of senescent fibro-adipogenic progenitors in progeria-aged muscle by senolytics rescues the function of muscle stem cells. J Cachexia Sarcopenia Muscle. 2022 Oct 11. doi: 10.1002/jcsm.13101. Epub ahead of print. PMID: 36218080.
Researchers from the Washington University School of Medicine in St. Louis show that the enzyme behind NMN biosynthesis – NAMPT – reverses metabolic derangements in obese mice.
By Jonathan D. Grinstein, Ph.D. www.nmn.com
Highlights
Intermittent fasting and caloric restriction improve metabolism and inflammation in mice and humans. That’s why both intermittent fasting and caloric restriction have been pursued as promising means by which to abate diseases of aging, overnutrition, and neurodegeneration. Calorie restriction abates aging and cardiometabolic disease by activating metabolic signaling pathways, including the nicotinamide adenine dinucleotide (NAD+) synthesis pathway.
Published in Nature Communications, researchers from Washington University School of Medicine in St. Louis show that NAMPT – an enzyme necessary for synthesizing the NAD+ precursor NMN – in the liver drives critical aspects of the fasting response. On the one hand, mice lacking NAMPT in liver cells exhibit defective thermal regulation during fasting and are sensitized to diet-induced glucose intolerance. On the other hand, increasing levels of NAMPT in liver cells induces fat browning, improved glucose balance, and attenuated high blood fat levels (dyslipidemia) in obese mice. This work shows that modulating NAD+ levels in liver cells can potentially mitigate fasting-associated disease.
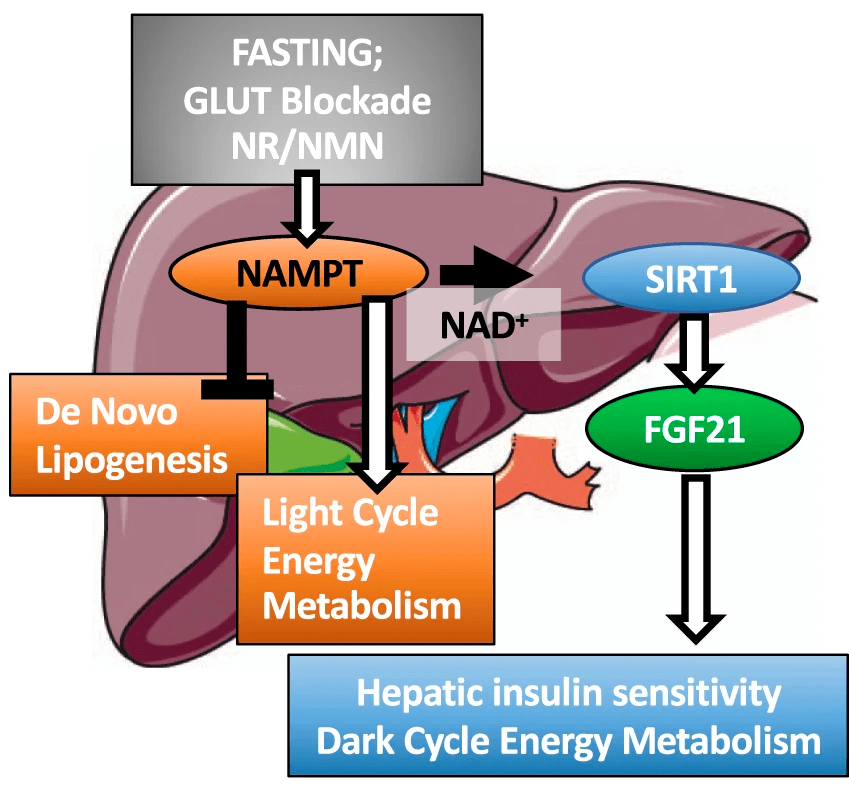
(Higgins et al., 2022 | Nature Communications) NAMPT in the liver balances energy metabolism. Liver cell NAMPT activation promotes light- and dark-cycle energy metabolism and liver insulin sensitivity while blocking the formation of fat (de novo lipogenesis). NAMPT can be activated by blocking glucose transporters (GLUT) or through supplementation with the NAD+ precursors nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN). NAMPT = nicotinamide phosphoribosyltransferase, SIRT1 = sirtuin 1, FGF21=fibroblast growth factor 21.
The Liver Is Central to Metabolic Regulation
The liver is uniquely positioned at the nexus between blood coming from the digestive system and blood being sent back to the heart to be pumped to the brain, limbs, and the rest of the body. Here, the liver can sense, coordinate, and transition between different states of metabolism that depend on feeding or the lack thereof (also known as fasting). Nutrient withdrawal creates an adapt-or-perish proposition for an organism in general and for liver cells in particular. The lack of sugar forces a reliance upon fat breakdown throughout the body, which gets metabolized in the liver. When this occurs, the liver needs to make several key adjustments. NAD+ synthesis unifies the adaptive responses to each of these stresses in the nutrient-restricted liver. Accordingly, NAD+ protects against diseases ranging from aging and neurodegeneration to diabetes and non-alcoholic fatty liver disease (NAFLD).
Liver NAMPT Is Necessary for Fasting Benefits
In this study, the Washington University of Medicine in St. Louis researchers dissect how NAMPT in liver cells modulates metabolic balance. Higgins and colleagues demonstrate that liver NAMPT mediates a key liver signaling cascade during fasting. The study shows that fasting and glucose transport inhibition upregulates NAMPT in the liver, while liver-specific NAMPT deficiency impairs several key fasting metabolic processes. More specifically, mice lacking NAMPT in the liver show defects in glucose metabolism, whereas viral-mediated increases in liver NAMPT enhance thermogenesis and glucose metabolism in diet-induced and genetically obese models. These findings show that liver NAMPT levels are essential to protect against diet-induced glucose intolerance and to elicit the metabolic benefits of fasting.
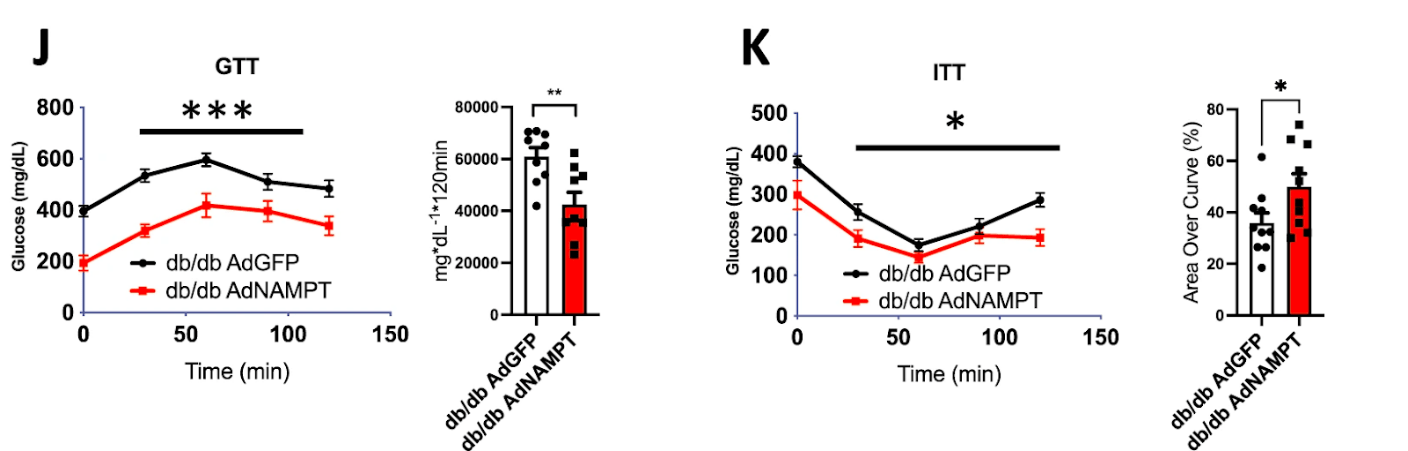
(Higgins et al., 2022 | Nature Communications) Liver NAMPT enhances glucose homeostasis in diabetic mice. Increased levels of NAMPT in obese, diabetic mice (db/db AdNAMPT) led to lower plasma glucose throughout both glucose and insulin tolerance testing, indicating improved glucose metabolism and insulin sensitivity.
Higgins and colleagues found that raising NAMPT levels in obese diabetic mice increased fat tissue browning markers. Fat tissue (usually white) turns brown when it is thermogenically active, meaning that it generates more heat while burning calories. Along these lines, mice with elevated liver NAMPT had higher thermogenesis levels than controls. Raising liver cell NAMPT induced increased heat production and gas exchange – more oxygen in and carbon dioxide out during breathing – in mice during both light- and dark cycles, whereas NAMPT deletion led to dark-cycle thermic defects. The data indicate that liver cell NAMPT signaling distinguishes between light- and dark-cycles when modulating energetic control.
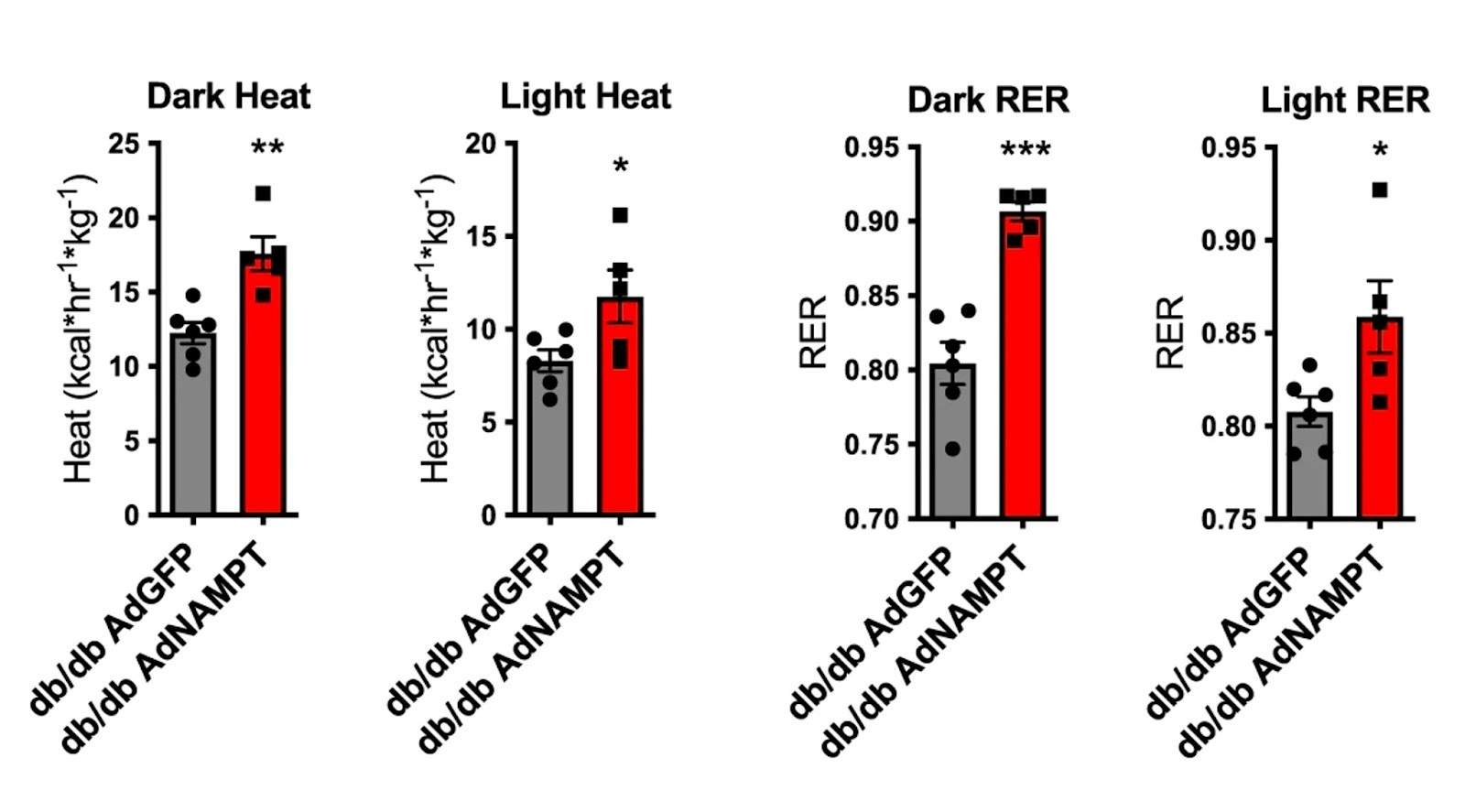
(Higgins et al., 2022 | Nature Communications) NAMPT induces fat tissue browning and thermogenesis in diabetic mice. To measure the role of NAMPT on thermogenesis and energy regulation, diabetic mice (db/db) were treated with a virus carrying NAMPT (AdNAMPT). Compared to controls, these mice exhibited significantly greater dark- and light-cycle thermogenesis and respiratory exchange rates (RER).
Higgins and colleagues conclude that NAMPT in the liver exerts broad fasting-mimetic effects downstream of generalized fasting and glucose transport inhibition in liver cells. This study suggests that metabolic disease can be intervened at the level of NAD+ biosynthesis via NMN. It is possible that NAMPT activation in the liver can fight against aging, obesity, and other fasting-responsive diseases. Interestingly, there are some compounds that activate NAMPT like SBI-797812, which turns NAMPT into a “super catalyst” that more efficiently generates NMN. Of note, mice dosed with SBI-797812 show elevation in liver NAD+. For all we know, this could be the basis for that long-sought-after miracle fasting pill.
Story Source Higgins CB, Mayer AL, Zhang Y, Franczyk M, Ballentine S, Yoshino J, DeBosch BJ. SIRT1 selectively exerts the metabolic protective effects of hepatocyte nicotinamide phosphoribosyltransferase. Nat Commun. 2022 Feb 28;13(1):1074. doi: 10.1038/s41467-022-28717