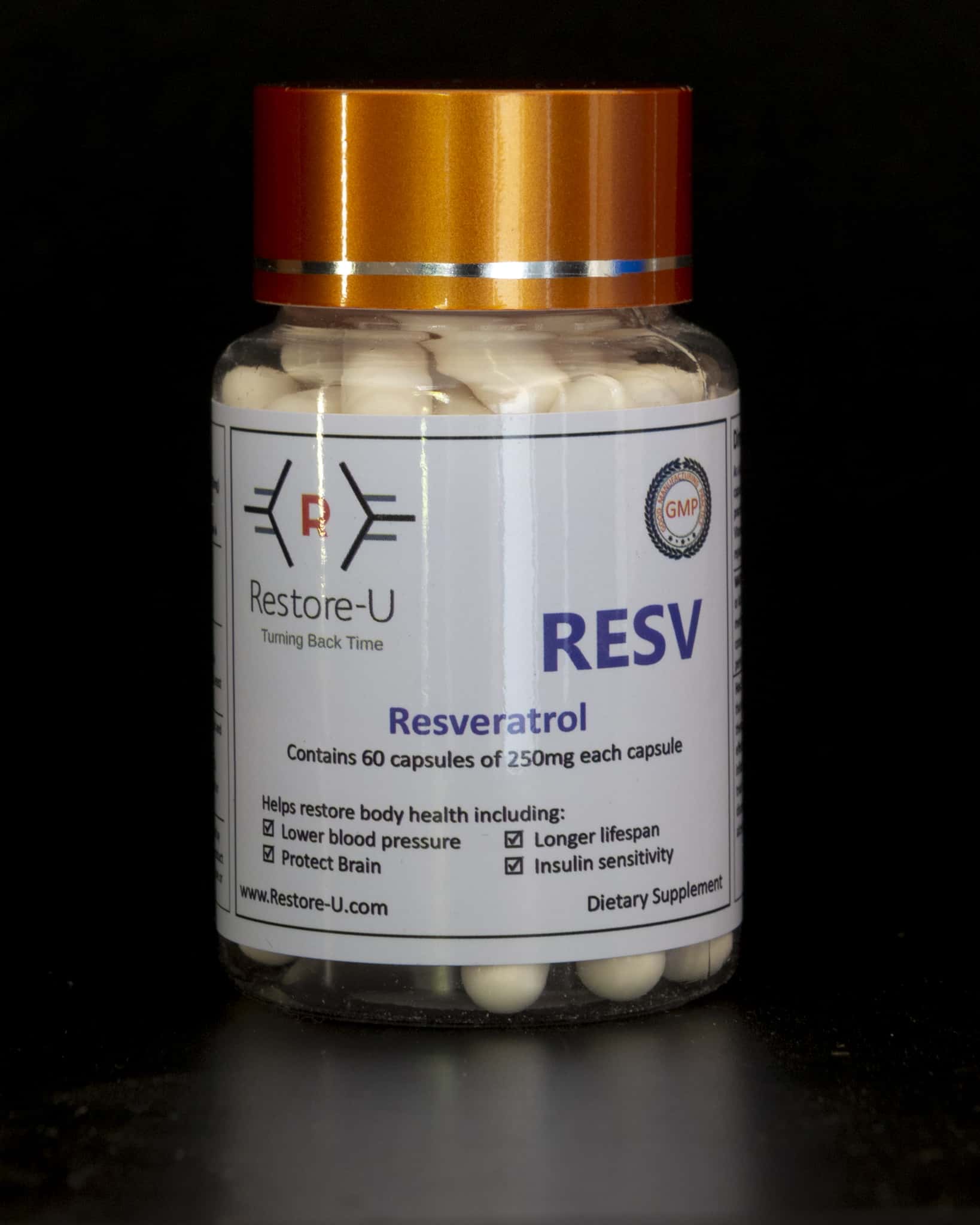
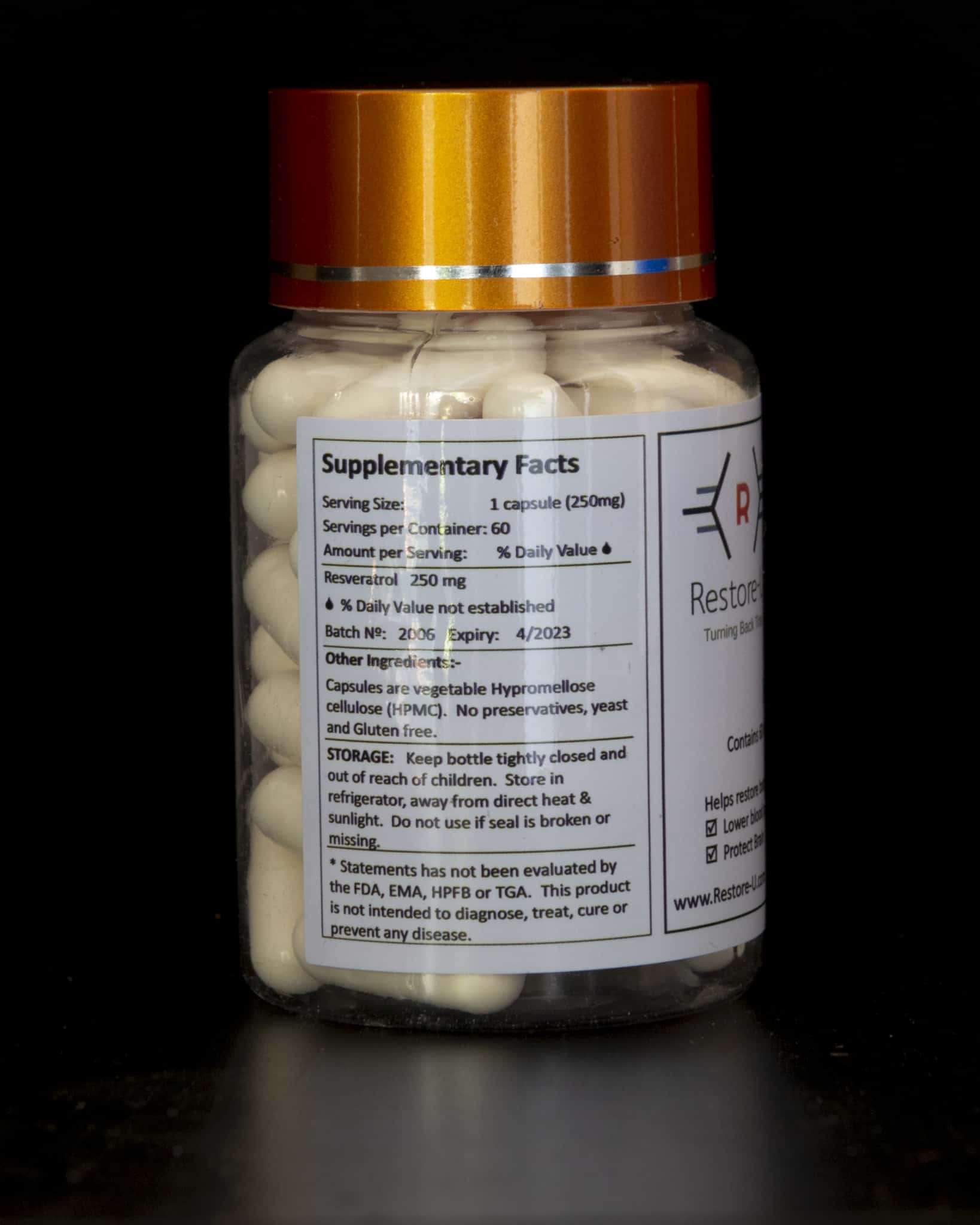
Description
What is Resveratrol (RSV)?
Resveratrol is a plant compound that acts like an antioxidant found in red wine, red grape skins, purple grape juice, mulberries, and in smaller amounts in peanuts. This compound tends to be concentrated mostly in the skins and seeds of grapes and berries.
Resveratrol is an anti-oxident, anti-aging, a NAD accelerator and a Sirtuin, which may enhance your longevity genes. Dr David Sinclair, refers to Resveratrol when paired with NMN, as the accelerator pedal to optimise energy and longevity.
Trans-Resveratrol represents the most highly absorbable form of Resveratrol and is the one most studied and referenced by research.
From the limited research in humans, most have focused on supplemental forms in concentrations higher than those you can get through food. Resveratrol is most commonly used for high cholesterol, cancer and heart disease and supporting healthy metabolic function.
It is well known for allegedly increasing lifespan. Resveratrol may help fight the ageing process and boost your overall health as you age. Discovered in 2003, by Dr David Sinclair, resveratrol is the polyphenol that has been long theorized to have anti-aging effects leading people to believe drinking a bit of wine every day may increase longevity. It triggers epigenetic changes that may delay aging and increase your energy levels.
It can, however, protect humans from heart disease and insulin resistance and may extend life by that mechanism. Resveratrol is most commonly used for high cholesterol, cancer, heart disease, and many other conditions. Combining resveratrol with Nicotinamide mononucleotide (NMN) creates a synergistic increase of effectiveness from both molecules.
How is Resveratrol Important to Anti Aging and NAD+?
Resveratrol has several key benefits that promotes longevity and supports NAD+ metabolism that makes it a perfect synergistic match for NMN and other NAD boosting supplements.
- A 2016 study showed Resveratrol promotes a healthy cardiovascular system by remodeling the gut bacteria to reduce the production of TMA and subsequent TMAO. TMAO interferes with the normal removal of cholesterol from the bloodstream by blocking its conversion to bile acids in the liver.
- Resveratrol speeds up the biosynthesis and recycling of NAD+ in our bodies by up-regulating the NMNAT gene, which is a bottleneck-enzyme responsible for the production of NAD+.
- Resveratrol can also directly, though selectively, turn on our “longevity genes” called Sirtuins, such as SIRT1, mimicking some benefits of caloric restriction and promoting mitochondrial biogenesis.
- Resveratrol aids in the protection of NAD+ by suppressing inflammatory cytokines that cause the expression of ectoenzyme CD38. CD38 the greatest destroyer of NAD+ in our bodies.
- Resveratrol is an AMPK activator which is a fatty acid oxidation pathway normally induced by exercise and is believed to be beneficial to longevity.
- Another recent study shows resveratrol may have the ability to rejuvenate senescent cells and elongate telomeres, ”making senescent cells not only look physically younger, but start to behave more like young cells and start dividing.”
- Resveratrol promotes general health and wellness in a multifaceted way that makes it indispensable as a nutritional supplement. “Antioxidant effect of resveratrol in the cardiovascular system”
Resveratrol and NAD+
- NAD+ is a coenzyme found in all living cells. It is essential for energy production and DNA repair. 1-3
- The most important NAD+ benefit is promoting rapid DNA repair and fuelling beneficial longevity proteins. 2,3,6,7
- Failure to repair damaged DNA can result in cell death or transformation into malignant or senescent states.
- Resveratrol favourably enhances the activation of cellular Sirtuin proteins. 8 NAD+ is required for our Sirtuins to function. 7,9,10
- As we age, NAD+ levels plummet, which impedes the ability of resveratrol to deliver its benefits. 11-14
- In animal studies, regenerative effects have been observed in the brain when NAD+ is restored. 29, 30
- Sleep quality deteriorates with normal aging in many people. Restoring youthful NAD+ levels in the brain may support a healthy circadian rhythm. 31
- Loss of NAD+ activity is linked to type II diabetes. In mice, administration of a NAD+ precursor restores insulin sensitivity and protects against the diabetic impact of a high-fat diet. 6,32,33
- Resveratrol has become a popular dietary supplement because of its ability to activate Sirtuin proteins in our cells. 34
- When Sirtuins are activated, the effect is delayed aging, which has been demonstrated in a wide spectrum of experimental models, including mammals. 35-44
- Sirtuins that are activated by resveratrol require NAD+ as their energy substrate. Loss of NAD+ impedes beneficial Sirtuin function. 7,10,45
- Younger people have high NAD+ levels that enable them to benefit from the Sirtuin-boosting effects of resveratrol.
- To improve the functionality of Sirtuin proteins, it makes sense for maturing individuals to boost their NAD+ levels.
NAD+ Benefits More Than Just Sirtuins
The favourable effect of resveratrol in promoting Sirtuin activity is well established. For Sirtuins to function properly, they must have sufficient NAD+ to fuel their activity. 7,10
Protecting against pathological aging, however, requires more than securing Sirtuin structure-function. We must also ensure the following two types of DNA damage are repaired:
- Single-strand DNA breaks occur often and are usually fixed by nutrients that most people supplement with today.
- Double-strand DNA breaks are more difficult to restore. Left unrepaired, double-strand breaks create cellular havoc that can lead to systemic degeneration.
A critical enzyme that repairs double-strand DNA breaks is PARP1. 46-48 For the PARP1 enzyme to function it requires lots of NAD+. 49,50
When it comes to protecting against cancer, a tumour suppressor called p53 protects against runaway cell propagation. NAD+ supports p53 activation to help thwart malignant transformation. 51-53
Magnitude of Daily DNA Damage
- A study analysed how many double-stranded DNA breaks occur per cell each day. The number turned out to be 10 DNA breaks per cell every day. 54
- Your cells require NAD+ to facilitate repair of DNA breaks. Sufficient NAD+ is needed for the PARP1 repair enzyme to function. 49,50,55
- Imagine the accumulated damage done by every dividing cell in your body undergoing ten double-stranded DNA breaks per day and NOT being repaired because your NAD+ is depleted from aging, or from outside abuse such as excess alcohol and toxic food ingestion.
- It helps explains many degenerative pathologies that occur as aging cells lose their NAD+.
- Repairing DNA breaks will probably go a long way towards preventing cells from turning malignant. That’s because NAD+ helps maintain activity of cell division regulators like p53. 51-53
Restoring Youthful Cell Functionality
- As we age, beneficial genes that support cell health “turn off” while detrimental genes overexpress.
- Nutrients like curcumin help suppress genes that generate system-wide inflammation. 56-59 Likewise, omega-3s 60-66 and vitamin D 67-70 favourably impact hundreds of genes that protect against degenerative illnesses.
- To reverse the accumulation of damage inflicted to cellular DNA, we should support the efficient function of PARP1 enzymes. PARP1 facilitates DNA repair via multiple mechanisms. Higher NAD+ cell levels enable PARP1 to function properly. 49,71,72
- Aging creates a chaotic environment in the brain that can make sound sleep difficult. 73 As DNA is repaired, we regain youthful cell functionality that can result in improved overall health.
- Combining resveratrol with more nicotinamide riboside supports healthy cellular NAD+ levels, 73 which are important to support anti-aging enzymes like PARP1 and BubR1. 71,74,75
- BubR1 is an enzyme that protects against chromosome instability. According to one study, sustained high-level expression of BubR1 “provides a unique opportunity to extend healthy lifespan”. 75
What benefit may I get from Resveratrol Supplements?
Researchers have suggested that consumption of resveratrol may potentially benefit numerous clinical conditions, including cancer, cardiovascular disease, obesity and related conditions including diabetes and non-alcoholic fatty liver disease, and neurological diseases. However, current evidence is insufficient to support a definitive place in therapy for resveratrol.
- Resveratrol Supplements May Help Lower Blood Pressure.
A 2015 review concluded that high doses may help reduce the pressure exerted on artery walls when the heart beats (Source).
- It Has a Positive Effect on Blood Fats
A 2016 study fed mice a high-protein, high-polyunsaturated fat diet and also gave them resveratrol supplements. Researchers found the average total cholesterol levels and body weight of the mice decreased, and their levels of “good” HDL cholesterol increased (Source).
In another study, participants were given grape extract that had been boosted with extra resveratrol. After six months of treatment, their LDL had gone down by 4.5% and their oxidized LDL had gone down by 20% compared to participants who took an unenriched grape extract or a placebo (Source).
- It Lengthens Lifespan in Certain Animals
Resveratrol’s ability to extend lifespan in different organisms has become a major area of research (Source).
There’s evidence that resveratrol activates certain genes that ward off the diseases of aging (Source).
It works to achieve this in the same way as calorie restriction, which has shown promise in lengthening lifespans by changing how genes express themselves (Source, Source).
- It Protects the Brain
Several studies have suggested that drinking red wine can help slow down age-related cognitive decline (Source, Source, Source, Source). This may partly be due to the antioxidant and anti-inflammatory activity of resveratrol.
It seems to interfere with protein fragments called beta-amyloids, which are crucial to forming the plaques that are a hallmark of Alzheimer’s disease (Source, Source).
- It May Increase Insulin Sensitivity
Resveratrol has been shown to have several benefits for diabetes, at least in animal studies.
These benefits include increasing insulin sensitivity and preventing complications from diabetes (Source, Source, Source, Source).
An explanation for how resveratrol works is that it may stop a certain enzyme from turning glucose into sorbitol, a sugar alcohol. When too much sorbitol builds up in people with diabetes, it can create cell-damaging oxidative stress (Source, Source).
Here are a few more benefits resveratrol may have for people with diabetes (Source):
- May protect against oxidative stress: Its antioxidant action may help protect against oxidative stress, which causes some of the complications of diabetes.
- Helps decrease inflammation: Resveratrol is thought to lessen inflammation, a key contributor to chronic diseases, including diabetes.
- Activates AMPK: This is a protein that helps the body metabolize glucose. Activated AMPK helps keep blood sugar levels low.
Resveratrol may even provide more benefits for people with diabetes than those who don’t have it. In one animal study, red wine and resveratrol were actually more effective antioxidants in rats with diabetes than in rats who didn’t have it (Source).
- It May Ease Joint Pain
Arthritis is a common affliction that leads to joint pain and loss of mobility (Source).
Plant-based supplements are being studied as a way to treat and prevent joint pain. When taken as a supplement, resveratrol may help protect cartilage from deteriorating (Source, Source).
Cartilage breakdown can cause joint pain and is one of the main symptoms of arthritis (Source).
One study injected resveratrol into the knee joints of rabbits with arthritis and found that these rabbits suffered less damage to their cartilage (Source).
Other research in test tubes and animals has suggested that the compound has potential to reduce inflammation and prevent damage to joints (Source, Source, Source, Source).
- Resveratrol May Suppress Cancer Cells
Resveratrol has been studied, especially in test tubes, for its ability to prevent and treat cancer. However, results have been mixed (Source, Source, Source).
In animal and test-tube studies, it has been shown to fight several kinds of cancer cells, including gastric, colon, skin, breast and prostate (Source, Source, Source, Source, Source).
Here’s how resveratrol may combat cancer cells:
- It may inhibit cancer cell growth: It may prevent cancer cells from replicating and spreading (Source).
- Resveratrol may change gene expression: It can change the gene expression in cancer cells to inhibit their growth (Source).
- It can have hormonal effects: Resveratrol may interfere with the way certain hormones are expressed, which may keep hormone-dependent cancers from spreading (Source).
What are the Side Effects of Resveratrol?
No major risks have been revealed in studies that have used resveratrol supplements. Healthy people seem to tolerate Resveratrol well (Source). Adverse events for lower doses and shorter courses of treatment have been rare. If you currently use medications, then you may want to check with a doctor before trying resveratrol.
How and what dosage do I take Resveratrol supplements?
Evidence from clinical studies is insufficient to provide comprehensive dosing guidelines. Single-dose studies suggest that a safe daily dosage for a person weighing 70 kg maybe 450 mg/day.
Dosages above 1 g/day appear to have been well tolerated in a short-term (2-week).
The lower end of supplementation tends to be for cardiovascular health, insulin sensitivity, and longevity for somebody who is otherwise unhealthy is 5-10mg daily.
For persons who are otherwise healthy, dosages between the range of 150-445mg have been used (with no clear indication for what is the optimal dose).
Supplementing for cerebral blood flow requires a dose in the 250-500mg range whereas supplementation for aromatase inhibition requires 500mg.
When taken by mouth, Resveratrol is likely safe when used in the amounts found in foods, such as yogurt. When taken in doses up to 1500 mg daily for up to 3 months, resveratrol is possible safe. Higher doses of up to 2000-3000 mg daily have been used safely for 2-6 months. However, these higher doses of resveratrol are more likely to cause stomach problems.
Reference links
Old human cells rejuvenated in breakthrough discovery on aging
Antioxidant effects of resveratrol in the cardiovascular system
Sirt1 is regulated by miR-135a and involved in DNA damage repair during mouse cellular reprogramming
Role of Resveratrol in Regulating Cutaneous Functions
Dose-dependency of resveratrol in providing health benefits
References
- Busso N, Karababa M, Nobile M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3(5):e2267.
- Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53. (References continued on page 12.)
- Satoh MS, Poirier GG, Lindahl T. NAD(+)-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993;268(8):5480-7.
- Available at: http://www.lifeextension.com/Magazine/2014/11/The-Youth-Restoring-Benefits-Of-NAD/Page-01. Accessed June 15, 2017.
- Trammell SA, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948.
- Canto C, Houtkooper RH, Pirinen E, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838-47.
- Canto C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64(1): 166-87.
- Lekli I, Ray D, Das DK. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2010;5(1):55-60.
- Landry J, Sutton A, Tafrov ST, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97(11):5807-11.
- Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38(5):349-59.
- Guest J, Grant R, Mori TA, et al. Changes in oxidative damage, inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9(1):e85335.
- Massudi H, Grant R, Braidy N, et al. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357.
- Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. Embo j. 2014;33(12):1321-40.
- Braidy N, Guillemin GJ, Mansour H, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6(4):e19194.
- Available at: http://www.lifeextension.com/Magazine/2008/2/Living-Longer-Healthier-Lives-With-Resveratrol/Page-01. Accessed June 15, 2015.
- Available at: http://www.lifeextension.com/magazine/2007/3/report_resveratrol/page-01. Accessed June 15, 2017.
- Available at: http://www.lifeextension.com/magazine/2006/7/report_longevity/Page-01. Accessed July 5, 2017.
- Dash S, Xiao C, Morgantini C, et al. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33(12):2895-901.
- Kennedy DO, Wightman EL, Reay JL, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91(6):1590-7.
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-71.
- Liu D, Pitta M, Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann N Y Acad Sci. 2008;1147:275-82.
- Min SW, Sohn PD, Cho SH, et al. Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front Aging Neurosci. 2013;5:53.
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem. 2006;96(2):305-13.
- Borradaile NM, Pickering JG. NAD(+), sirtuins, and cardiovascular disease. Curr Pharm Des. 2009;15(1):110-7.
- Hsu CP, Oka S, Shao D, et al. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105(5):481-91.
- Pillai VB, Sundaresan NR, Kim G, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285(5):3133-44.
- Frederick DW, Loro E, Liu L, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016;24(2):269-82.
- Zhou CC, Yang X, Hua X, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173(15):2352-68.
- Zhao Y, Guan YF, Zhou XM, et al. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke. 2015;46(7):1966-74.
- Wang S, Xing Z, Vosler PS, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39(9):2587-95.
- Imai S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta. 2010;1804(8):1584-90.
- Yoshino J, Mills KF, Yoon MJ, et al. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528-36.
- Trammell SA, Weidemann BJ, Chadda A, et al. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep. 2016;6:26933.
- Higashida K, Kim SH, Jung SR, et al. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11(7):e1001603.
- Rehan L, Laszki-Szczachor K, Sobieszczanska M, et al. SIRT1 and NAD as regulators of ageing. Life Sci. 2014;105 (1-2):1-6.
- Imai S-i, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017.
- Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154(2):430-41.
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570-80.
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998-6003.
- Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30(7):271-86.
- Satoh A, Brace CS, Rensing N, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416-30.
- Satoh A, Imai S. Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat Commun. 2014;5:4211.
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305(5686):1010-3.
- Schmeisser K, Mansfeld J, Kuhlow D, et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693-700.
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253-95.
- Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332(6036):1443-6.
- Hochegger H, Dejsuphong D, Fukushima T, et al. Parp-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. Embo j. 2006;25(6):1305-14.
- Paddock MN, Buelow BD, Takeda S, et al. The BRCT domain of PARP-1 is required for immunoglobulin gene conversion. PLoS Biol. 2010;8(7):e1000428.
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19(17):1951-67.
- Siegel C, McCullough LD. NAD+ depletion or PAR polymer formation: which plays the role of executioner in ischaemic cell death? Acta Physiol (Oxf). 2011;203(1):225-34.
- Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. Embo j. 2002;21(10):2383-96.
- Pfister NT, Yoh KE, Prives C. p53, DNA damage, and NAD+ homeostasis. Cell Cycle. 2014;13(11):1661-2.
- McLure KG, Takagi M, Kastan MB. NAD+ modulates p53 DNA binding specificity and function. Mol Cell Biol. 2004;24(22):9958-67.
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181-211.
- El Ramy R, Magroun N, Messadecq N, et al. Functional interplay between Parp-1 and SirT1 in genome integrity and chromatin-based processes. Cell Mol Life Sci. 2009;66(19):3219-34.
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane). J Biol Chem. 1995;270(42):24995-5000.
- Ranjan D, Chen C, Johnston TD, et al. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling. J Surg Res. 2004;121(2):171-7.
- Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004;1030:434-41.
- Kumar A, Dhawan S, Hardegen NJ, et al. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55(6):775-83.
- Bouwens M, van de Rest O, Dellschaft N, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90(2):415-24.
- Weaver KL, Ivester P, Seeds M, et al. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem. 2009;284(23):15400-7.
- Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131(4):1129-32.
- Gillies PJ, Bhatia SK, Belcher LA, et al. Regulation of inflammatory and lipid metabolism genes by eicosapentaenoic acid-rich oil. J Lipid Res. 2012;53(8):1679-89.
- Thomas J, Thomas CJ, Radcliffe J, et al. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed Res Int. 2015;2015:172801.
- Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. 2010;88(10):2091-102.
- Kitajka K, Puskas LG, Zvara A, et al. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc Natl Acad Sci U S A. 2002;99(5):2619-24.
- Chakraborti CK. Vitamin D as a promising anticancer agent. Indian J Pharmacol. 2011;43(2):113-20.
- Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4(3):e103.
- Bao BY, Yao J, Lee YF. 1alpha, 25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis. 2006;27(9):1883-93.
- Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One. 2013;8(3):e58725.
- Schiewer MJ, Knudsen KE. Transcriptional roles of PARP1 in cancer. Mol Cancer Res. 2014;12(8):1069-80.
- Everson CA, Henchen CJ, Szabo A, et al. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37(12):1929-40.
- Mouchiroud L, Houtkooper RH, Auwerx J. NAD(+) metabolism, a therapeutic target for age-related metabolic disease. Critical reviews in biochemistry and molecular biology. 2013;48(4):10.3109/10409238.2013.789479.
- North BJ, Rosenberg MA, Jeganathan KB, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. The EMBO Journal. 2014;33(13):1438-53.
- Baker DJ, Dawlaty MM, Wijshake T, et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol. 2013;15(1):96-102.
- Halicka HD, Zhao H, Li J, et al. Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3. Aging (Albany NY). 2012;4(4):270-8.
- Available at: http://www.lifeextension.com/magazine/2013/8/The-Overlooked-Importance-of-Vitamin-D-Receptors/Page-01. Accessed June 16, 2017.
- Fleet JC, DeSmet M, Johnson R, et al. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441(1):61-76.
- Ames BN. A role for supplements in optimizing health: the metabolic tune-up. Arch Biochem Biophys. 2004;423(1): 227-34.
- Wei Q, Shen H, Wang LE, et al. Association between low dietary folate intake and suboptimal cellular DNA repair capacity. Cancer Epidemiol Biomarkers Prev. 2003;12(10):963-9.
- Duthie SJ. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis. 2011;34(1):101-9.
- Basten GP, Duthie SJ, Pirie L, et al. Sensitivity of markers of DNA stability and DNA repair activity to folate supplementation in healthy volunteers. Br J Cancer. 2006;94(12):1942-7.
- Choi SW, Kim YI, Weitzel JN, et al. Folate depletion impairs DNA excision repair in the colon of the rat. Gut. 1998;43(1):93-9.
- Kruman, II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22(5):1752-62.
- Sadik NA, Shaker OG. Dietary folate suppresses DMH-induced colon carcinogenesis in a rat model and affects DMH-induced expression of four DNA repair enzymes. Nutr Cancer. 2012;64(8):1196-203.
- Surjana D, Halliday GM, Damian DL. Role of Nicotinamide in DNA Damage, Mutagenesis, and DNA Repair. Journal of Nucleic Acids. 2010;2010:13.
- Hong MY, Lupton JR, Morris JS, et al. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev. 2000;9(8):819-26.
- Ghorbanihaghjo A, Safa J, Alizadeh S, et al. Protective effect of fish oil supplementation on DNA damage induced by cigarette smoking. J Health Popul Nutr. 2013;31(3):343-9.
- Stephenson JA, Al-Taan O, Arshad A, et al. The multifaceted effects of omega-3 polyunsaturated Fatty acids on the hallmarks of cancer. J Lipids. 2013;2013:261247.
- Alzoubi K, Khabour O, Hussain N, et al. Evaluation of vitamin B12 effects on DNA damage induced by pioglitazone. Mutat Res. 2012;748(1-2):48-51.
- Sweetman SF, Strain JJ, McKelvey-Martin VJ. Effect of antioxidant vitamin supplementation on DNA damage and repair in human lymphoblastoid cells. Nutr Cancer. 1997;27(2):122-30.
- Cooke MS, Evans MD, Podmore ID, et al. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998;439(3):363-7.
- Tomasetti M, Alleva R, Borghi B, et al. In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. Faseb j. 2001;15(8):1425-7.
- Song Y, Leonard SW, Traber MG, et al. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009;139(9):1626-31.
- Hartwig A. Role of magnesium in genomic stability. Mutat Res. 2001;475(1-2):113-21.
- Mahabir S, Wei Q, Barrera SL, et al. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis. 2008;29(5):949-56.
- de Rosa V, Erkekoglu P, Forestier A, et al. Low doses of selenium specifically stimulate the repair of oxidative DNA damage in LNCaP prostate cancer cells. Free Radic Res. 2012;46(2):105-16.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71-83.
- Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175(5):1952-61.
- Tan X, Zhao C, Pan J, et al. In vivo non-enzymatic repair of DNA oxidative damage by polyphenols. Cell Biol Int. 2009;33(6):690-6.
- Katiyar SK, van Steeg H, Sharma SD. Abstract 1875: Dietary grape seed proanthocyanidins induce rapid repair of DNA damage via nucleotide excision repair genes in preventing UV-induced immunosuppression. Cancer Research. 2010;70(8 Supplement):1875-.
- Mansouri E, Khorsandi L, Abedi HA. Antioxidant effects of proanthocyanidin from grape seed on hepatic tissue injury in diabetic rats. Iran J Basic Med Sci. 2014;17(6):460-4.
- Roy M, Sinha D, Mukherjee S, et al. Curcumin prevents DNA damage and enhances the repair potential in a chronically arsenic-exposed human population in West Bengal, India. Eur J Cancer Prev. 2011;20(2):123-31.
- Mukherjee S, Roy M, Dey S, et al. A Mechanistic Approach for Modulation of Arsenic Toxicity in Human Lymphocytes by Curcumin, an Active Constituent of Medicinal Herb Curcuma longa Linn. J Clin Biochem Nutr. 2007;41(1):32-42.
- Astley SB, Elliott RM, Archer DB, et al. Increased cellular carotenoid levels reduce the persistence of DNA single-strand breaks after oxidative challenge. Nutr Cancer. 2002;43(2):202-13.
- Lorenzo Y, Azqueta A, Luna L, et al. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30(2):308-14.
- Astley SB, Elliott RM, Archer DB, et al. Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: repair balance in human lymphocytes. Br J Nutr. 2004;91(1):63-72.
- Chou YC, Chu CH, Wu MH, et al. Dietary intake of vitamin B(6) and risk of breast cancer in Taiwanese women. J Epidemiol. 2011;21(5):329-36.
- Le Marchand L, White KK, Nomura AM, et al. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2195-201.
- Available at: http://www.lifeextension.com/Magazine/2014/6/The-2013-SENS-Foundation-Conference/Page-01. Accessed June 16, 2017.
- Available at: http://www.lifeextension.com/Magazine/2016/7/Age-Reversal-Research-at-Harvard-Medical-School/Page-01. Accessed June 16, 2017.
- Thomas P, Wang YJ, Zhong JH, et al. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat Res. 2009;661(1-2):25-34.
- Shu L, Khor TO, Lee JH, et al. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. Aaps j. 2011;13(4):606-14.
- Bistulfi G, Vandette E, Matsui S, et al. Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biol. 2010;8:6.
- Duthie SJ, Narayanan S, Sharp L, et al. Folate, DNA stability and colo-rectal neoplasia. Proc Nutr Soc. 2004;63(4):571-8.
- James SJ, Pogribny IP, Pogribna M, et al. Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J Nutr. 2003;133(11 Suppl 1):3740s-7s.
- Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, et al. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63(22):7799-806.
- Fernandez-Garcia NI, Palmer HG, Garcia M, et al. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene. 2005;24(43):6533-44.
- Stefanska B, Salame P, Bednarek A, et al. Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br J Nutr. 2012;107(6):781-90.
- Kelley MR, Logsdon D, Fishel ML. Targeting DNA repair pathways for cancer treatment: what’s new? Future Oncol. 2014;10(7):1215-37.
- Park HJ. Chemotherapy induced peripheral neuropathic pain. Korean J Anesthesiol. 2014;67(1):4-7.
- Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm. 2014;71(1):19-25.
- Visovsky C, Collins M, Abbott L, et al. Putting evidence into practice: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11(6):901-13.
- Stubblefield MD, McNeely ML, Alfano CM, et al. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(8 Suppl):2250-60.
- Feedback from chemotherapy patients using nicotinamide riboside dietary supplements. Life Extension Wellness Specialist Report. June 2017.
- Hamity MV, White SR, Walder RY, et al. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain. 2017;158(5): 962-72.
- Available at: http://www.health.harvard.edu/heart-health/cancer-treatments-may-harm-the-heart. Accessed June 16, 2017.
- Available at: https://www.texasoncology.com/cancer-treatment/side-effects-of-cancer-treatment/long-term-side-effects/cardiac-toxicity. Accessed June 16, 2017.
- Svoboda M, Poprach A, Dobes S, et al. Cardiac toxicity of targeted therapies used in the treatment for solid tumours: a review. Cardiovasc Toxicol. 2012;12(3):191-207.
- Marinko T, Dolenc J, Bilban-Jakopin C. Cardiotoxicity of concomitant radiotherapy and trastuzumab for early breast cancer. Radiol Oncol. 2014;48(2):105-12.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-98.
- Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011;2011:317659.
- Cortes EP, Gupta M, Chou C, et al. Adriamycin cardiotoxicity: early detection by systolic time interval and possible prevention by coenzyme Q10. Cancer Treat Rep. 1978;62(6):887-91.
- Iarussi D, Auricchio U, Agretto A, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15 Suppl:s207-12.
- Albini A, Pennesi G, Donatelli F, et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14-25.
- Rusciani L, Proietti I, Paradisi A, et al. Recombinant interferon alpha-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: a 3-year trial with recombinant interferon-alpha and 5-year follow-up. Melanoma Res. 2007;17(3):177-83.
- Folkers K, Brown R, Judy WV, et al. Survival of cancer patients on therapy with coenzyme Q10. Biochem Biophys Res Commun. 1993;192(1):241-5.
- Lockwood K, Moesgaard S, Folkers K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem Biophys Res Commun. 1994;199(3):1504-8.
- Lockwood K, Moesgaard S, Hanioka T, et al. Apparent partial remission of breast cancer in ‘high risk’ patients supplemented with nutritional antioxidants, essential fatty acids and coenzyme Q10. Mol Aspects Med. 1994;15 Suppl:s231-40.
- Smyth JF, Bowman A, Perren T, et al. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol. 1997;8(6):569-73.
- Lissoni P, Chilelli M, Villa S, et al. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;35(1):12-5.
- Israel L, Hajji O, Grefft-Alami A, et al. Vitamin A augmentation of the effects of chemotherapy in metastatic breast cancers after menopause. Randomized trial in 100 patients. Ann Med Interne (Paris). 1985;136(7):551-4.
- Zou YH, Liu XM. Effect of astragalus injection combined with chemotherapy on quality of life in patients with advanced non-small cell lung cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23(10):733-5.
- Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25(6):669-74.
- Pace A, Giannarelli D, Galie E, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010;74(9):762-6.
- Rouse K, Nwokedi E, Woodliff JE, et al. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann Surg. 1995;221(4):420-6.
- Kim SR, Jo SK, Kim SH. Modification of radiation response in mice by ginsenosides, active components of Panax ginseng. In Vivo. 2003;17(1):77-81.
- Xie FY, Zeng ZF, Huang HY. Clinical observation on nasopharyngeal carcinoma treated with combined therapy of radiotherapy and ginseng polysaccharide injection. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(5):332-4.
- Kiremidjian-Schumacher L, Roy M, Glickman R, et al. Selenium and immunocompetence in patients with head and neck cancer. Biol Trace Elem Res. 2000;73(2):97-111.
- Malmberg KJ, Lenkei R, Petersson M, et al. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clin Cancer Res. 2002;8(6):1772-8.
- Kumar B, Jha MN, Cole WC, et al. D-alpha-tocopheryl succinate (vitamin E) enhances radiation-induced chromosomal damage levels in human cancer cells, but reduces it in normal cells. J Am Coll Nutr. 2002;21(4):339-43.
- Kennedy RS, Konok GP, Bounous G, et al. The use of a whey protein concentrate in the treatment of patients with metastatic carcinoma: a phase I-II clinical study. Anticancer Res. 1995;15(6b):2643-9.
- Todorova VK, Harms SA, Kaufmann Y, et al. Effect of dietary glutamine on tumor glutathione levels and apoptosis-related proteins in DMBA-induced breast cancer of rats. Breast Cancer Res Treat. 2004;88(3):247-56.
- Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215(2):129-40.
- Hillman GG, Wang Y, Kucuk O, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model Mol Cancer Ther. 2004;3(10):1271-9.
- Yashar CM, Spanos WJ, Taylor DD, et al. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol Oncol. 2005;99(1):199-205.
- Kotowski U, Heiduschka G, Brunner M, et al. Radiosensitization of head and neck cancer cells by the phytochemical agent sulforaphane. Strahlenther Onkol. 2011;187(9):575-80.
- He H, Zhou X, Wang Q, et al. Does the couse of astragalus-containing chinese herbal prescriptions and radiotherapy benefit to non-small-cell lung cancer treatment: a meta-analysis of randomized trials. Evid Based Complement Alternat Med. 2013;2013:426207.
- Kwan ML, Greenlee H, Lee VS, et al. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2011;130 (1):195-205.


Reviews
There are no reviews yet.