Abstract
Nicotinamide adenine dinucleotide (NAD), the cell’s hydrogen carrier for redox enzymes, is well known for its role in redox reactions. More recently, it has emerged as a signalling molecule. By modulating NAD+-sensing enzymes, NAD+ controls hundreds of key processes from energy metabolism to cell survival, rising and falling depending on food intake, exercise, and the time of day.
NAD+ levels steadily decline with age, resulting in altered metabolism and increased disease susceptibility. Restoration of NAD+ levels in old or diseased animals can promote health and extend lifespan, prompting a search for safe and efficacious NAD-boosting molecules that hold the promise of increasing the body’s resilience, not just to one disease, but to many, thereby extending healthy human lifespan
Background
Nicotinamide adenine dinucleotide (NAD) is one of the most important and interesting molecules in the body. It is required for over 500 enzymatic reactions and plays key roles in the regulation of almost all major biological processes. Above all, it may allow us to lead healthier and longer lives.
NAD+ is one of the most abundant molecules in the human body, required for approximately 500 different enzymatic reactions and present at about three grams in the average person. Though it was once considered a relatively stable molecule, NAD+ is now known to be in a constant state of synthesis, degradation, and recycling, not only in the cytoplasm where most research is focused, but also within major organelles including the nucleus, Golgi, and peroxisomes.
With the exception of neurons, mammalian cells cannot import NAD+, so they must synthesize it either de novo by the kynure- nine pathway from tryptophan or forms of vitamin B3 such as nicotinamide (NAM) or nicotinic acid (NA) (Figure 1).
To maintain NAD+ levels, most NAD+ is recycled via salvage pathways rather than generated de novo. The majority of NAD+ is salvaged from NAM, the product of CD38 and the PARPs or from the various forms of niacin taken up in the diet, including NAM, NA, nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN).
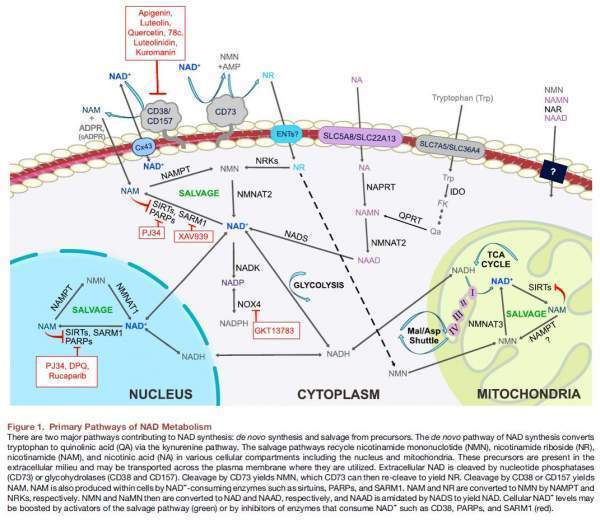
NAD+-Responsive Signalling Pathways
In mammals, the two main NAD+-responsive signalling protein families are the sirtuins and the PARP.
Sirtuins regulate a wide variety of mammalian proteins involved in processes that include mitochondrial metabolism, inflammation, meiosis, autophagy, circadian rhythms, and apoptosis.
PARPs are required for numerous cellular processes, including DNA repair and transcriptional regulation hyperactivation in response to DNA damage, causing depleting the cell of NAD+, most critically in the mitochondria, and thereby inducing apoptosis
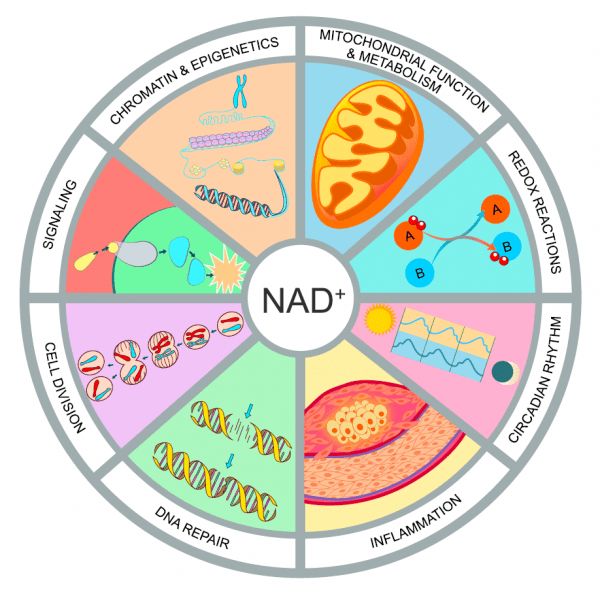
Figure 2. Hallmarks of NAD Homeostasis
NAD+ is not merely a redox co-factor, it is also a key signalling molecule that controls cell function and survival in response to environmental changes such as nutrient intake and cellular damage.
Fluctuations in NAD impact mitochondrial function and metabolism, redox reactions, circadian rhythm, immune response and inflammation, DNA repair, cell division, protein-protein signalling, chromatin, and epigenetics.
NAD+ Precursors
It is now known that NAD+ levels decline with age and that raising levels back up to or even above baseline provides a surprising number of health benefits in a wide range of organisms, from yeast to rodents. The first evidence that NAD+ boosting could increase lifespan came from studies our laboratory performed in yeast cells over 15 years ago.
In Drosophila, overexpression of the Pnc1 homolog D-NAAM extends mean lifespan by 30%, indicating that the upregulation of NAD synthesis might be a conserved longevity mechanism. (Conserved means similarity among different organisms and species).
NR and NMN are soluble and orally bioavailable endogenous molecules, making them the molecules of choice for animal experiments and human clinical trials.
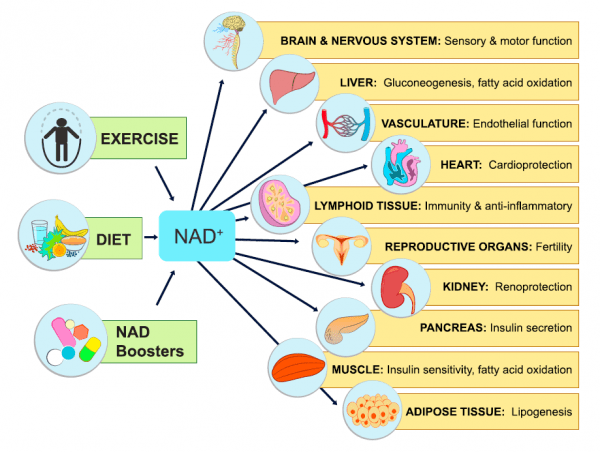
Figure 3. Physiological Effects of NAD Boosting Molecules
NAD+ levels steadily fall as we age, leading to a decline in the function of cells and organs. By raising NAD
NAD+ boosters can have profound effects on the health and survival of mammals.
Increases in NAD, NAD+ promote cognitive and sensory function, gluconeogenesis in liver, lipogenesis in adipose tissue, insulin secretion in pancreas, and insulin sensitivity in muscle. NAD also promotes endothelial cell proliferation and protects against cardio and cerebrovascular disease.
NAD regulates immune function and inflammation and protects against acute injury in kidney. NAD promotes and extends fertility in both males and females, ostensibly by activation of sirtuins.
Effects of NAD Boosters on Physiology and Health in Mouse Models
The initial discovery that genetically upregulating NAD+ biosynthesis can increase the stress resistance and lifespan of yeast cells and Drosophila prompted investigation of NAD+ boosters in rodents, both wild type and disease models, often with dramatic effects (Figure 3).
Liver Function
Key enzymes in NAD+ signalling pathways are known to protect the liver from fat accumulation, fibrosis, and insulin resistance, which are related to the development of fatty liver diseases, such as NAFLD and NASH.
SIRT1 and its downstream targets PGC-1a, PSK9, and SREBP1 maintain mitochondrial function cholesterol transport, and fatty acid homeostasis SIRT2 controls gluconeo- genesis by deacetylating phosphoenol- pyruvatecarboxykinase; SIRT3 regulates OXPHOS, fatty acid oxidation, ketogenesis, and defense against oxidative stress; and SIRT6 controls gluconeogenesis.
(NAFLD means Non-alcoholic fatty liver disease & NASH stands for Non-Alcoholic SteatoHepatitis)
Given the critical nature of these pathways in the liver, the maintenance of NAD+ levels is imperative for optimal organ function. As a result of obesity and aging, levels of NAMPT decline and CD38 levels increase, leading to a 2-fold decrease in steady-state NAD+ levels by mid-age.
Raising NAD+ levels back to those of young or lean mice has been particularly effective at preventing and treating obesity, alcoholic steatohepatitis, and NASH, while improving glucose homeostasis and mitochondrial dysfunction.
NAD+ boosting appears to not only improve the health of the liver, but also increase its capacity for regeneration and protect it against hepatotoxicity.
Kidney Function
Several lines of evidence indicate that reduced levels of NAD+ in aged kidneys and a corresponding decrease in sirtuin activity are largely responsible for reduced kidney function and resilience with age.
Consistent with this, activation of SIRT1 and SIRT3 by NAD+ supplementation protects against high-glucose-induced kidney mesangial cell hypertrophy.
Skeletal Muscle Function
Old mice have increased markers of muscle atrophy and inflammation as well as impaired insulin signalling and insulin-stimulated glucose uptake compared to young wild-type mice.
Treatment of old mice with NAD+ precursors, such as NR and NMN, dramatically improves muscle function.
Cardiac Function
NAD+ levels are critical for normal heart function and recovery from injury. Of all the NAD+-dependent signalling proteins, SIRT3 seems to be the most important in this context.
SIRT3 knockout mice develop fibrosis and cardiac hypertrophy as early as 13 months of age, which is further exacerbated by the loss of SIRT3 in aged and hypertrophic hearts, a decline that can be reversed by treatment with NMN.
NMN treatment either 30 min before ischemia (500 mg/kg, i.p.) or repetitive administration just before and during reperfusion provides marked protection against pressure overload and ischemia-reperfusion injury, reducing infarct size by as much as 44%.
Treatment with NAD+ precursors also improves heart function in old MDX mice with cardiomyopathy, improves mitochondrial and cardiac function in a mouse model for iron deficiency-induced heart failure, and restores cardiac function to near-normal levels in a mouse mode of Friedreich’s ataxia (FRDA) cardiomyopathy.
Endothelial and Vascular Function
Cardiovascular and cerebrovascular diseases contribute to the greatest decline in quality of life after 65 and are directly responsible for about one-third of all deaths.
Treatment of old mice with NMN (300 mg/kg/day for 8 weeks) restores carotid artery endothelium-dependent dilation, a measure of endothelial function, while reducing aortic pulse wave velocity and elastic artery stiffness
The performance of most organs and tissues is critically dependent on an abundant, fully functional microcapillary network that maintains a supply of oxygen, exchanges heat and various nutrients, and removes the waste products of metabolism.
Yet one of the most profound changes to the body as it ages is a decline in the number and function of endothelial cells that line the vasculature. Treatment of mice with NMN (500 mg/kg/day in water for 28 days) improves bloodflow and increases endurance in elderly mice by promoting SIRT1-dependent increases in capillary density.
Thus repleting NAD+ levels in the vascular endothelium is an attractive approach to increasing mobility in the elderly and treating conditions exacerbated by decreased blood flow, such as ischemia-reperfusion injury, slow wound healing, liver dysfunction, and muscle myopathies.
DNA Repair and Cancer
Because NAD+ is involved in so many aspects of cancer biology, from mitochondrial activity to cell survival, there are a variety of ways it could be used in the clinic. The rationale for reducing NAD+ levels in tumors is that they will be less able to repair DNA damage, thereby increasing their sensitivity to chemothera- peutic agents.
The other approach to treating cancer has been to increase NAD+ levels, the rationale being that an excess of NAD+ will boost mitochondrial respiration and downregulate glycolysis, counteracting the Warburg metabolism that cancer cells prefer. Increased NAD+ would also boost the activity of SIRT1 and SIRT6, both of which can inhibit tumors by down-regulating b catenin signalling and glycolysis. Long-term studies of wild-type mice, however, failed to provide any evidence of increased tumor size or number.
Interestingly, overexpression of NMNAT3 raises mitochondrial NAD+ and inhibits the growth of glioblastoma cells, and supplementation with NA or NAM inhibits tumor growth and multi-organ metastasis in SCID mice.
Immunity and Inflammation
There is a growing body of evidence that NAD+ precursors can have anti-inflammatory effects.
Treatment of 24-month-old mice with NMN for 1 week reduced the expression of inflammation markers such as TNF-a and IL-6 in skeletal muscle. Similarly, NR significantly reduced inflammation in a mouse model of ataxia telangiectasia (AT) autoimmunity.
NAM has been effective in the treatment of various inflammatory skin conditions, reduces the area of infiltration and demyelination in experimental autoimmune encephalomyelitis mouse models, and prevents photo-immunosuppression and photo-carcinogenesis.
Neuronal Function
The neuroprotective effects of NAD+ precursors were first revealed by a study of middle cerebral artery-induced ischemia, where treatment with NAM reduced the extent of infarct in Wistar rats, a finding recently replicated by treatment with NMN.
Numerous studies have reinforced the view that NAD+ levels are key to neuronal function and survival.
NMN preserves hippocampus-dependent spatial memory after forebrain ischemia and reduces edema, oxidative stress, inflammation, and neuronal death in a mouse collagenase-induced intracerebral hemorrhage model.
In addition to protecting damaged neurons, NAD+ precursors have shown promise in delaying the effects of several neurode-generative diseases.
In models of Alzheimer’s disease (AD), NR and NMN treatments improve cognition and synaptic plasticity in mice and rats.
Both NR and NMN improve motor function and memory in worm and mouse models of AT (ataxia telangiectasia).
NAD-boosting regimens prevent and in some cases can reverse neuronal degeneration associated with hearing loss, prion toxicity, retinal damage, traumatic brain injury (TBI), and peripheral neuropathy.
Aging and Longevity
Total NAD+ levels were once considered extremely stable. Recently, however, it has become clear that a steady decline in total NAD+ levels over time is a natural part of life for all species, from yeast to humans.
This decline, along with the decreased activity of NAD+ signalling proteins, is believed to be one of the major reasons organisms, including humans, age.
Given that budding yeast were first shown to live longer when the salvage pathway was upregulated, it was only appropriate that NAD+ precursors were first shown to extend lifespan in this species. In mice, raising NAD+ levels has been effective in delaying progeroid (accelerated aging) phenotypes and extending life-span in various models. In the case of the BubR1 mouse, median lifespan was extended by 58% percent and maximum by 21%,
Treatment of old (20-month-old) mice with NR extended their lifespan by nearly 5%, even when started at an age where few treatments work well, with the exception of rapamycin. Increased lifespan was also associated with a variety of physiological benefits including improved mitochondrial function and preservation of stem cell function.
Though NMN or other NAD+ boosters have not yet been tested for their effects on murine lifespan, some mice have been dosed for long periods. For example, starting at 5 months, NMN was administered to mice for over a year. Treated mice had increased activity, improved insulin sensitivity and lipid profiles, improved vision, and greater bone density. Taken together, these results, along with the various health benefits and age-reversal activities listed above, support the possibility of using NAD+ boosters as therapeutics against a broad range of age-associated diseases and possibly as a way to delay aging and age-related physical decline.
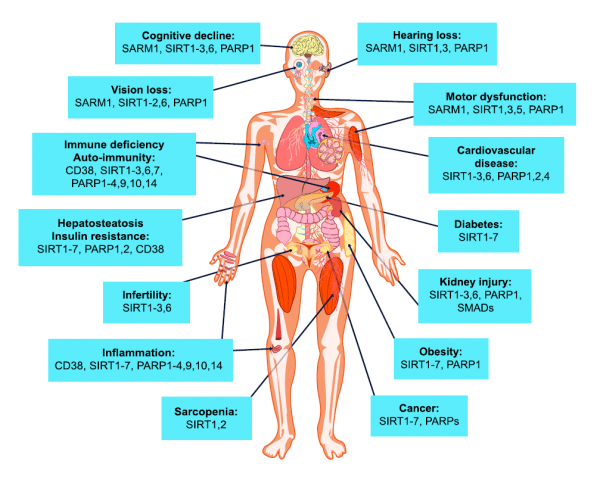
Figure 4. Potential Impact of NAD+ Boosters on Human Health via NAD+ Signaling Pathways
A decline in NAD+ during aging is believed to be a major cause of disease and disability, such as hearing and vision loss, as well as cognitive and motor dysfunction, immune deficiencies, autoimmunity, and dysregulation of the inflammatory response leading to arthritis, metabolic dysfunction, and cardiovascular disease.
In mouse models, NAD+ boosters prevent or treat a variety of different diseases, prompting a search for NAD+ boosters that are safe and effective as drugs to treat both rare and common diseases and, potentially, aging itself.
Perspective
Given that NAD was discovered over 100 years ago, it is surprising how little we know about its biology, pharmacology, and role in human disease. Though there is much to learn, we know this much: NAD+ boosters seem relatively safe and have a remarkable ability to prevent and treat diseases.
To read the study in full: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6342515/
Luis Rajman, Karolina Chwalek, and David A. Sinclair
Published: February 2018